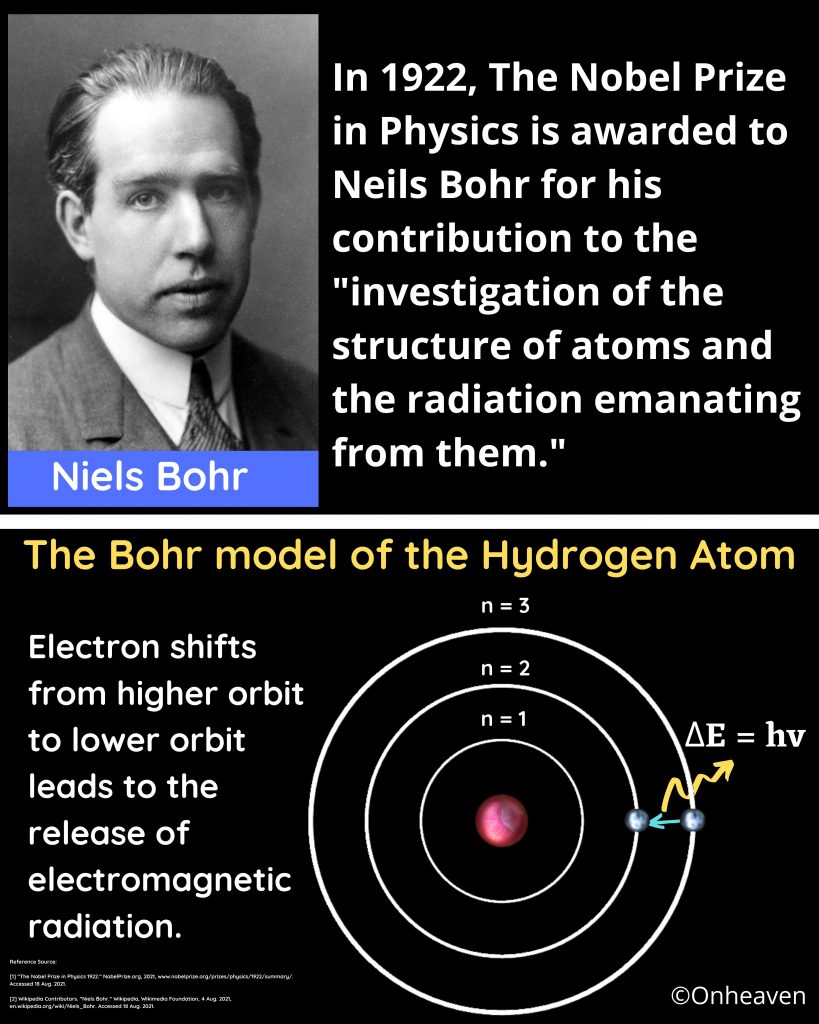
What does Bohr’s model explain? The four principles of Bohr’s model and Structure.
What does Bohr’s model explain?
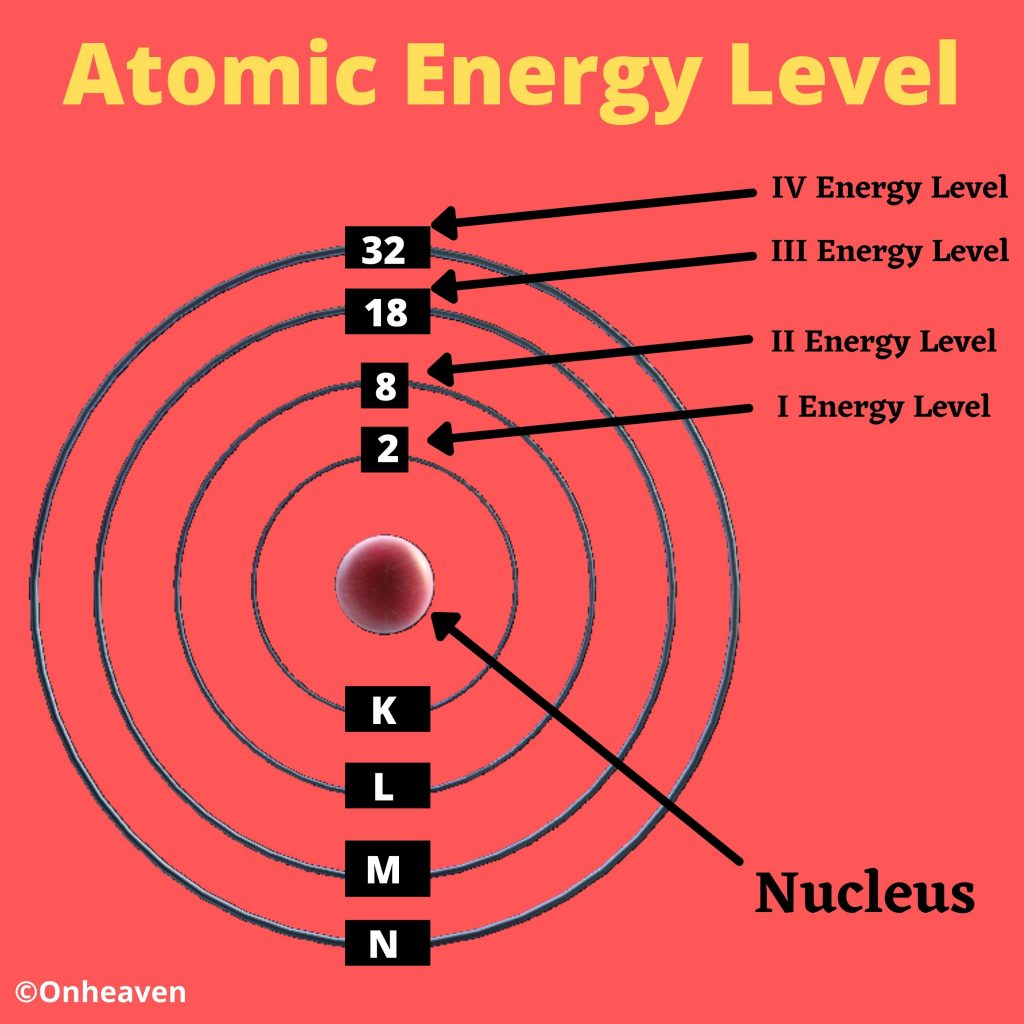
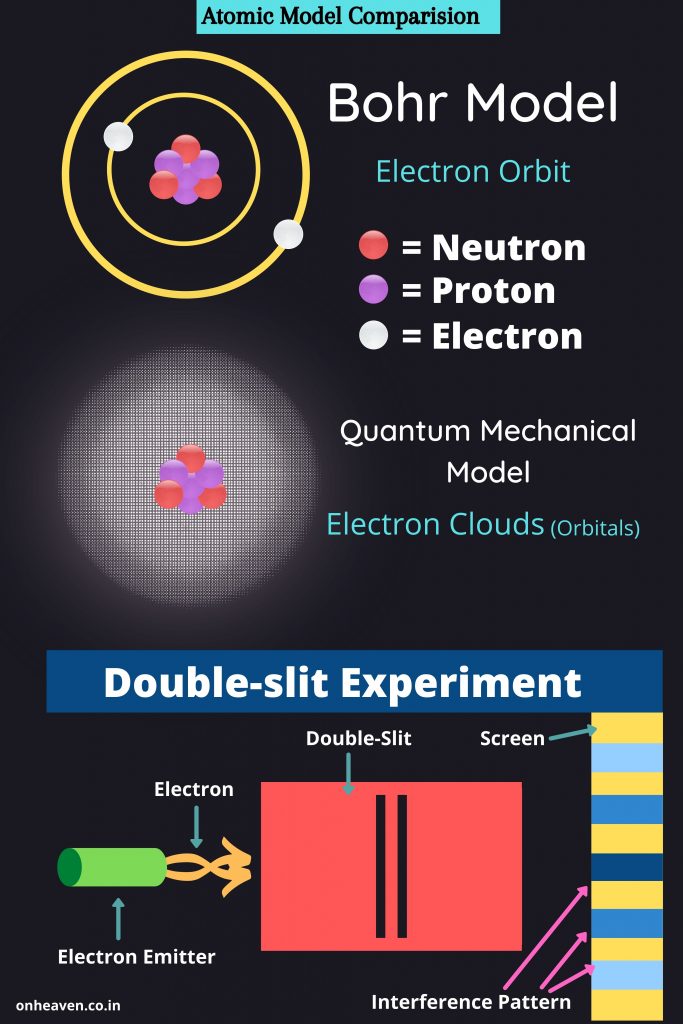
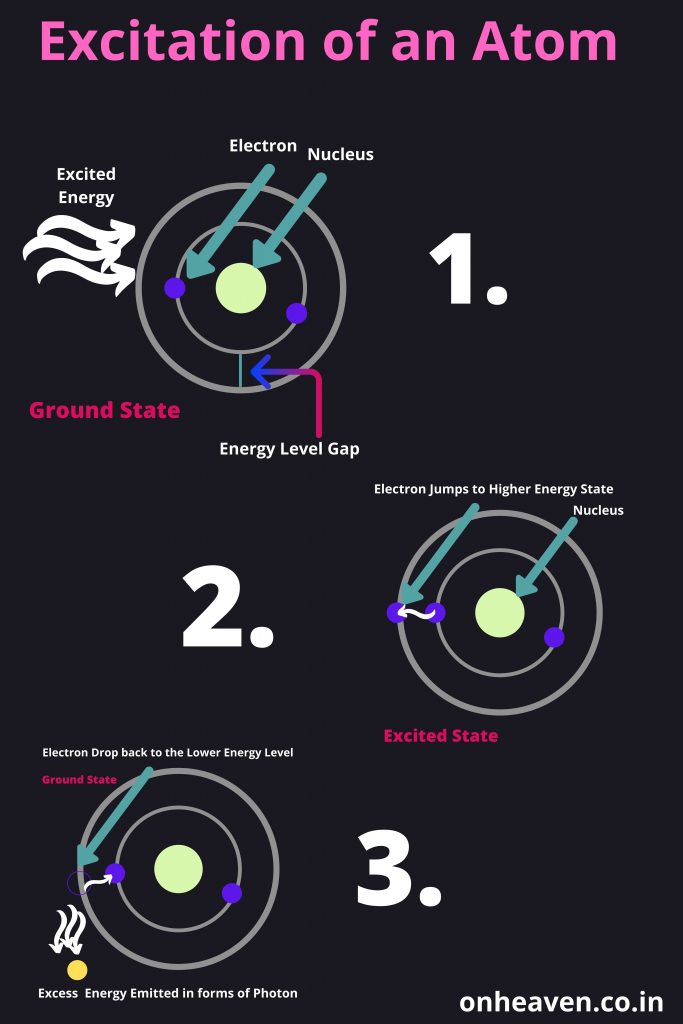
The Bohr model demonstrate that the electrons in an atoms are in orbits of different energy around the nucleus.
What are the four principles of Bohr’s model?
Here are the four principles, in a nutshell
- Electron occupies certain orbits in the nucleus.
- Each orbit has an energy associated with it.
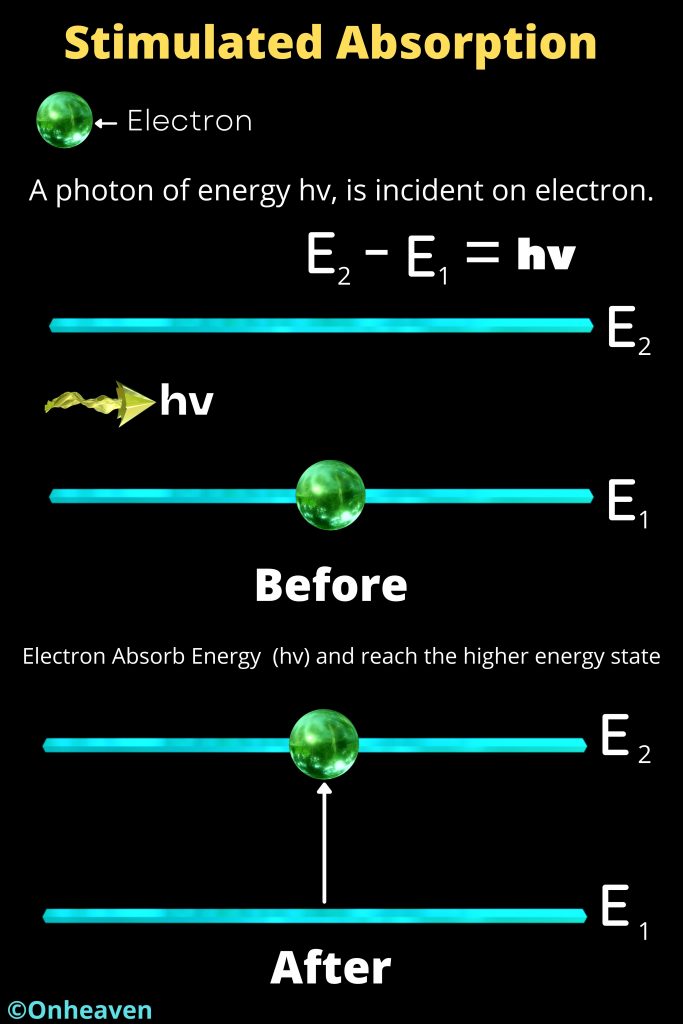
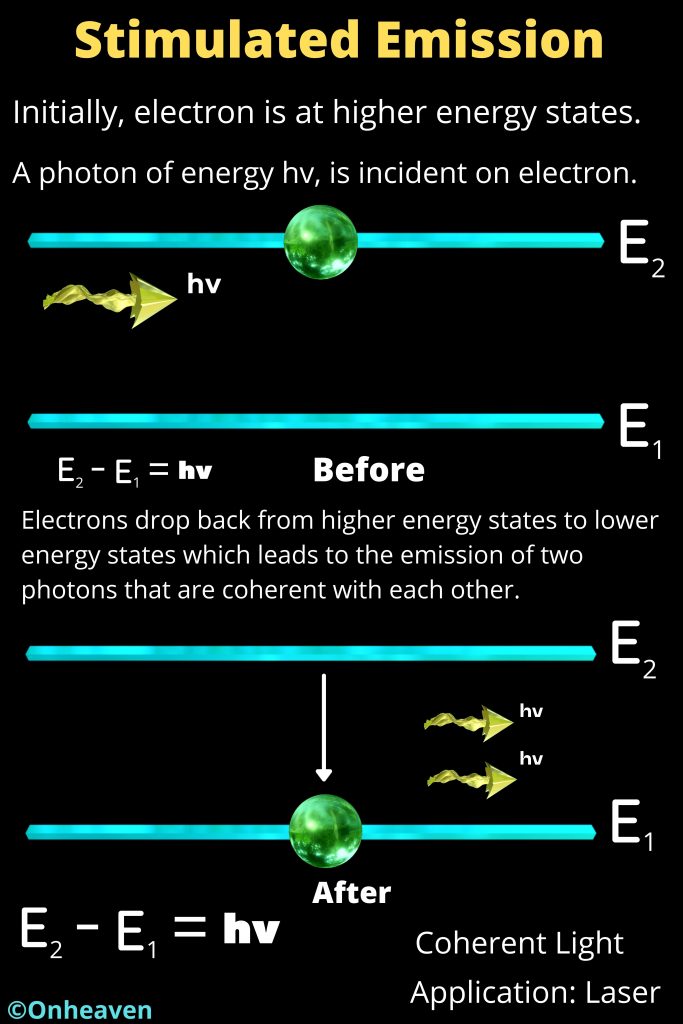
- Energy emitted when an electron moves from higher energy orbit to lower energy orbit, and the energy absorbed when an electron move from lower energy orbit to higher energy orbit.
- Energy and frequency of light emitted or Absorbed in the form of energy, can be calculated by the difference between the orbitals energy.
What is Bohr’s model of the atom called?
Bohr’s model of an atom is also referred as Planetary model.
What does Bohr’s model look like?
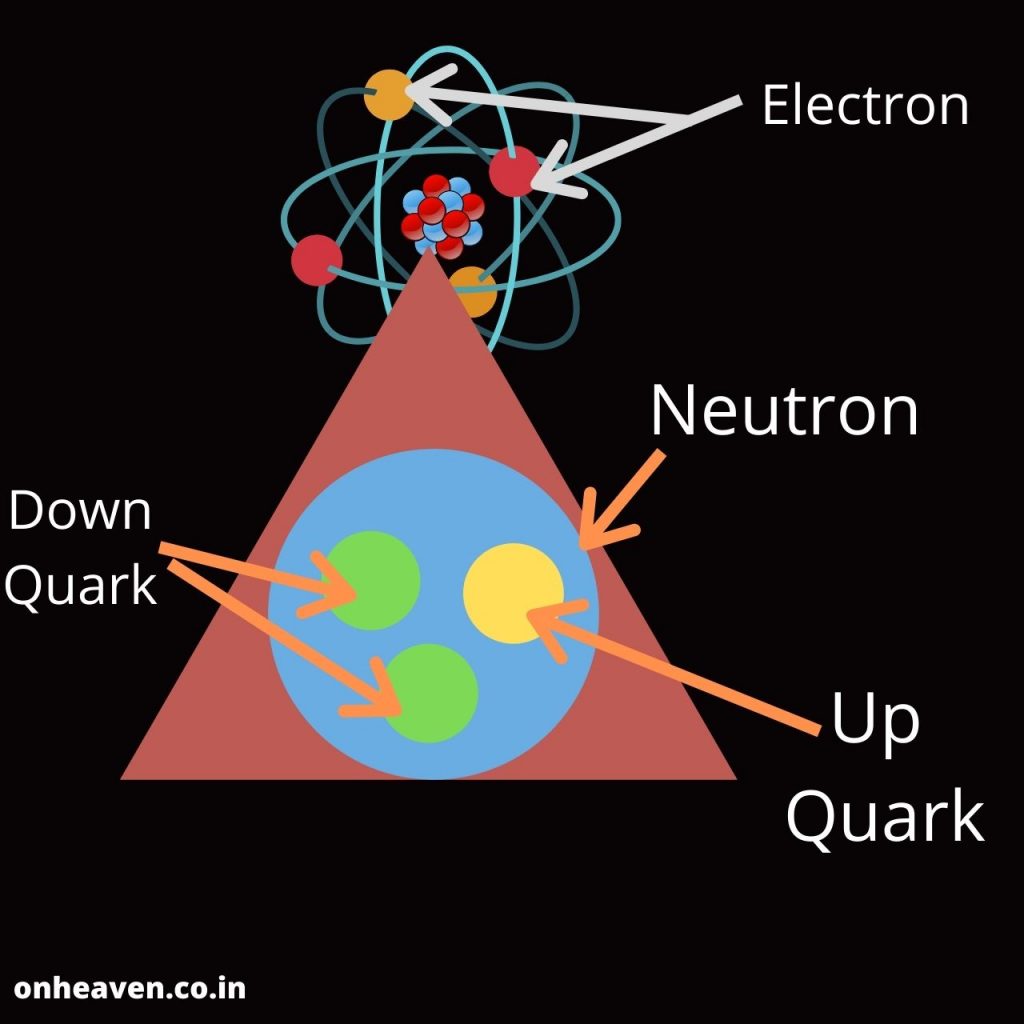
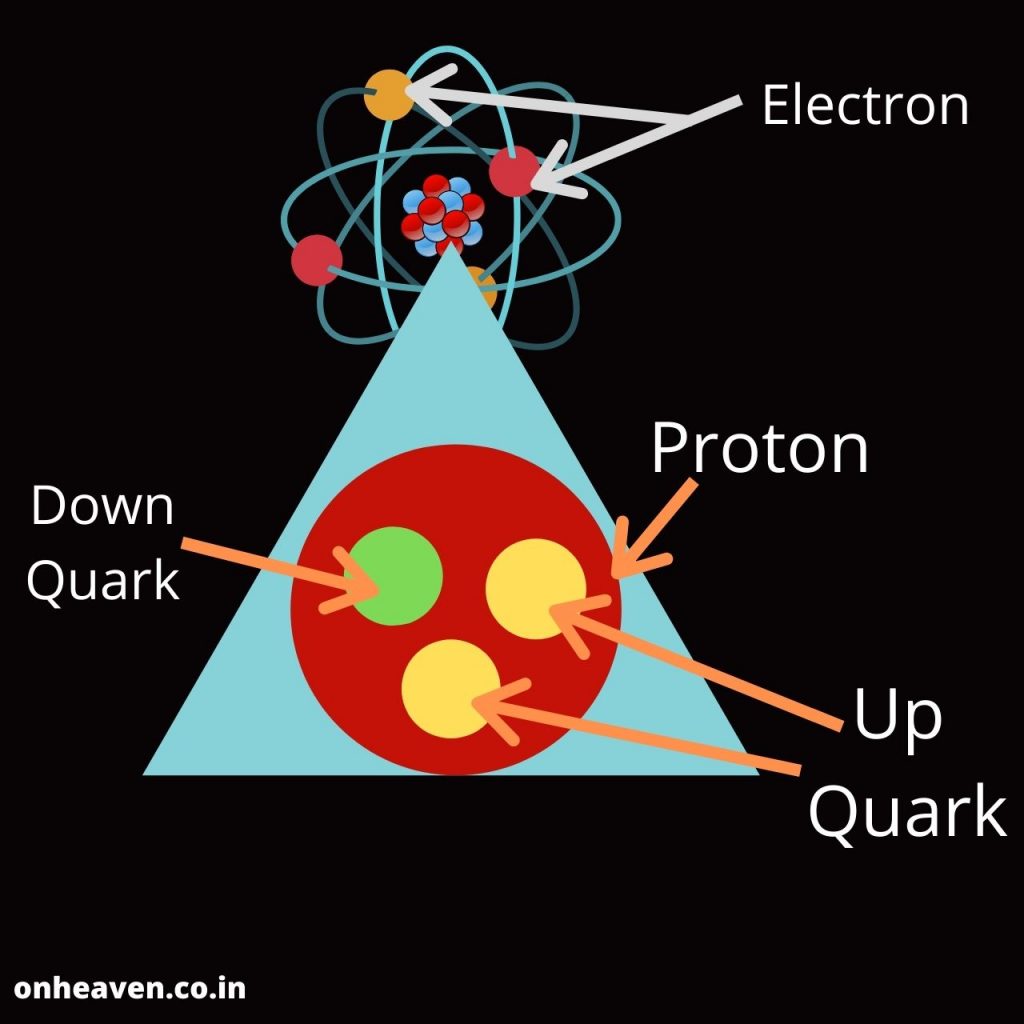
Bohr’s model depicts the structure of an atom as a small positively charged nucleus surrounded by electrons. Here is the pictorial representation of Bohr’s atomic model which is given below,
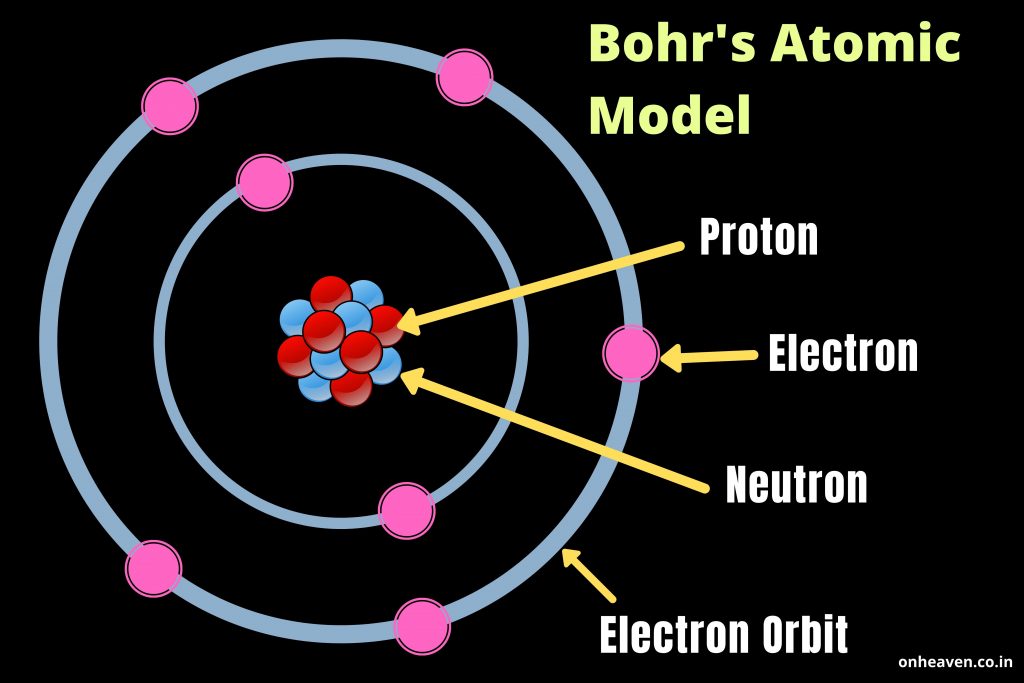
Reference Source:
[1] “Atomic Structure: The Bohr Model – Dummies.” Dummies, 2016, www.dummies.com/education/science/chemistry/atomic-structure-the-bohr-model/. Accessed 30 June 2021.
[2] “Development of the Atomic Theory.” Abcte.org, 2021, www.abcte.org/files/previews/chemistry/s1_p6.html. Accessed 30 June 2021.
[3] “Bohr’s Atomic Model | Chemistry for Non-Majors.” Lumenlearning.com, 2021, courses.lumenlearning.com/cheminter/chapter/bohrs-atomic-model/. Accessed 30 June 2021.
[4] “The Bohr Model | Introduction to Chemistry.” Lumenlearning.com, 2021, courses.lumenlearning.com/introchem/chapter/the-bohr-model/. Accessed 30 June 2021.
Recent Articles (Science Gallery)
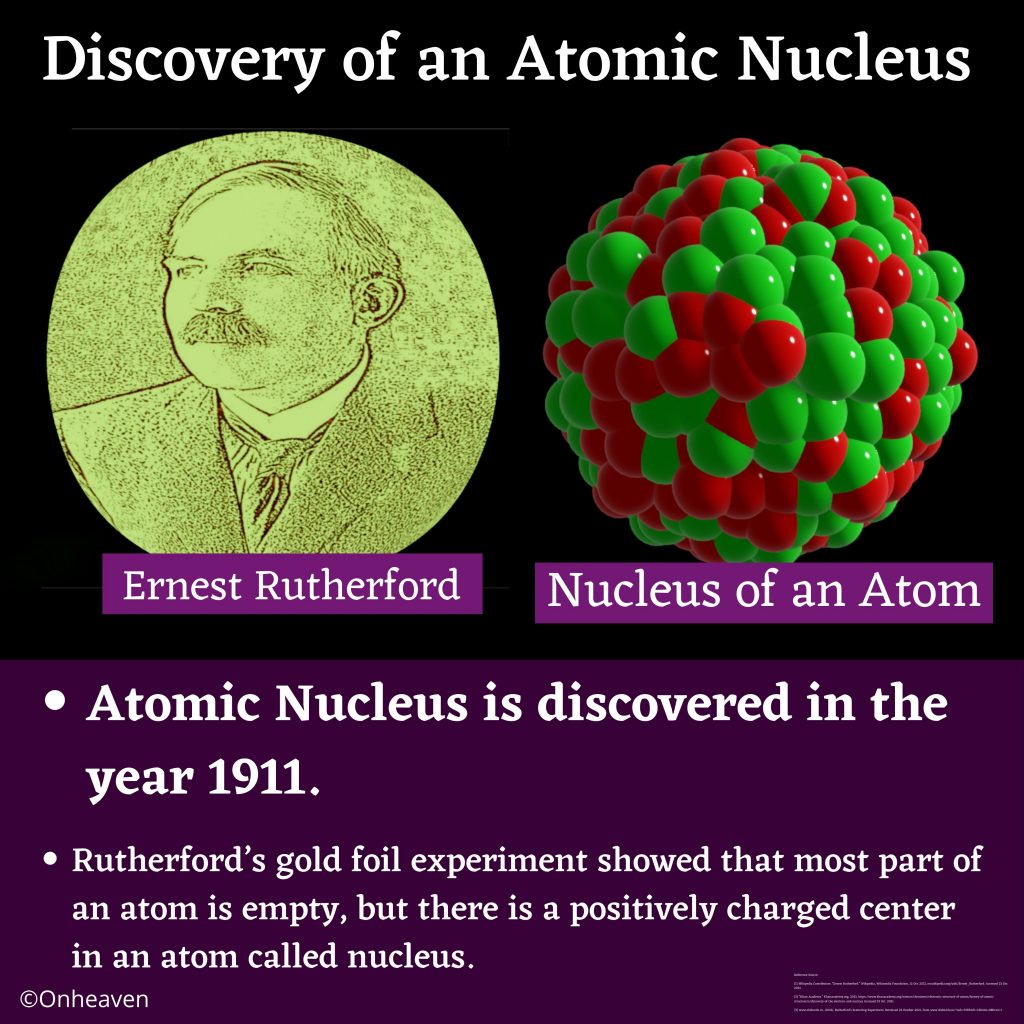

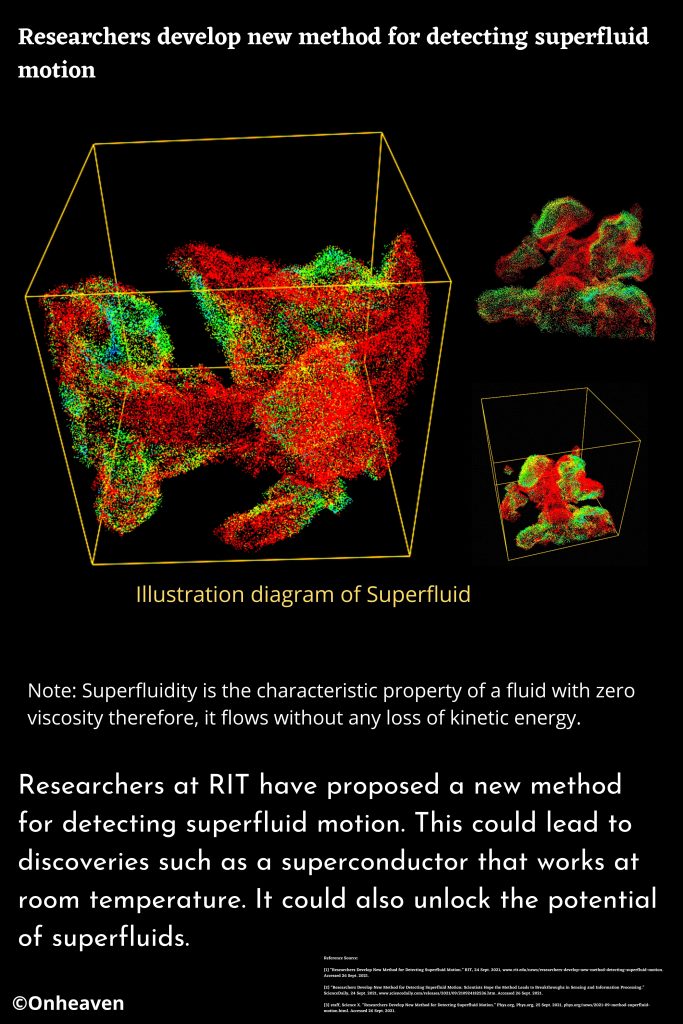
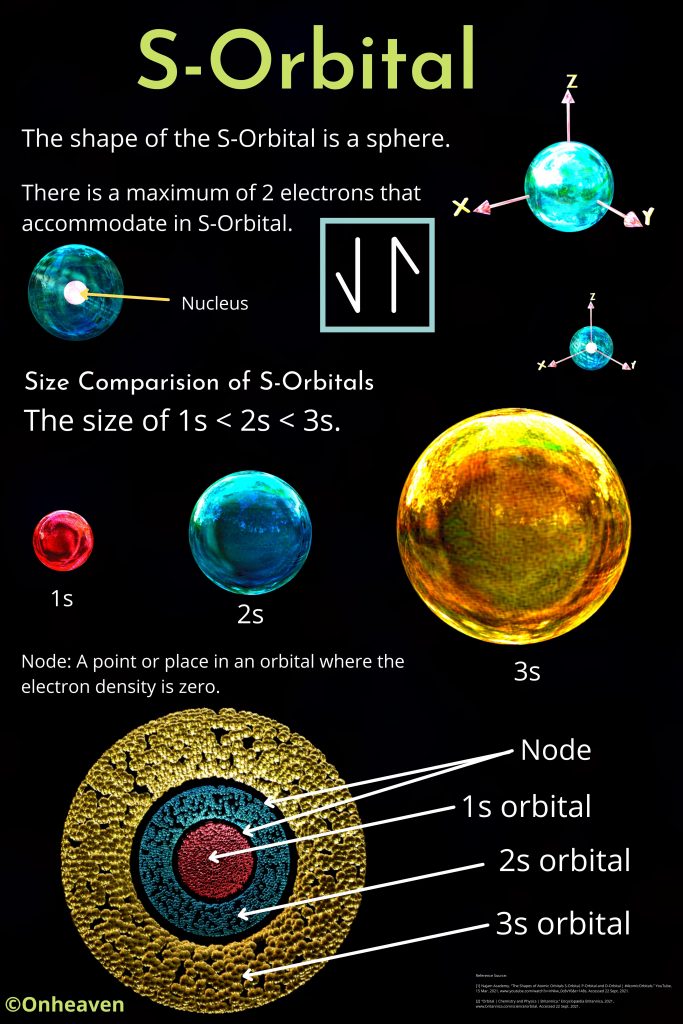
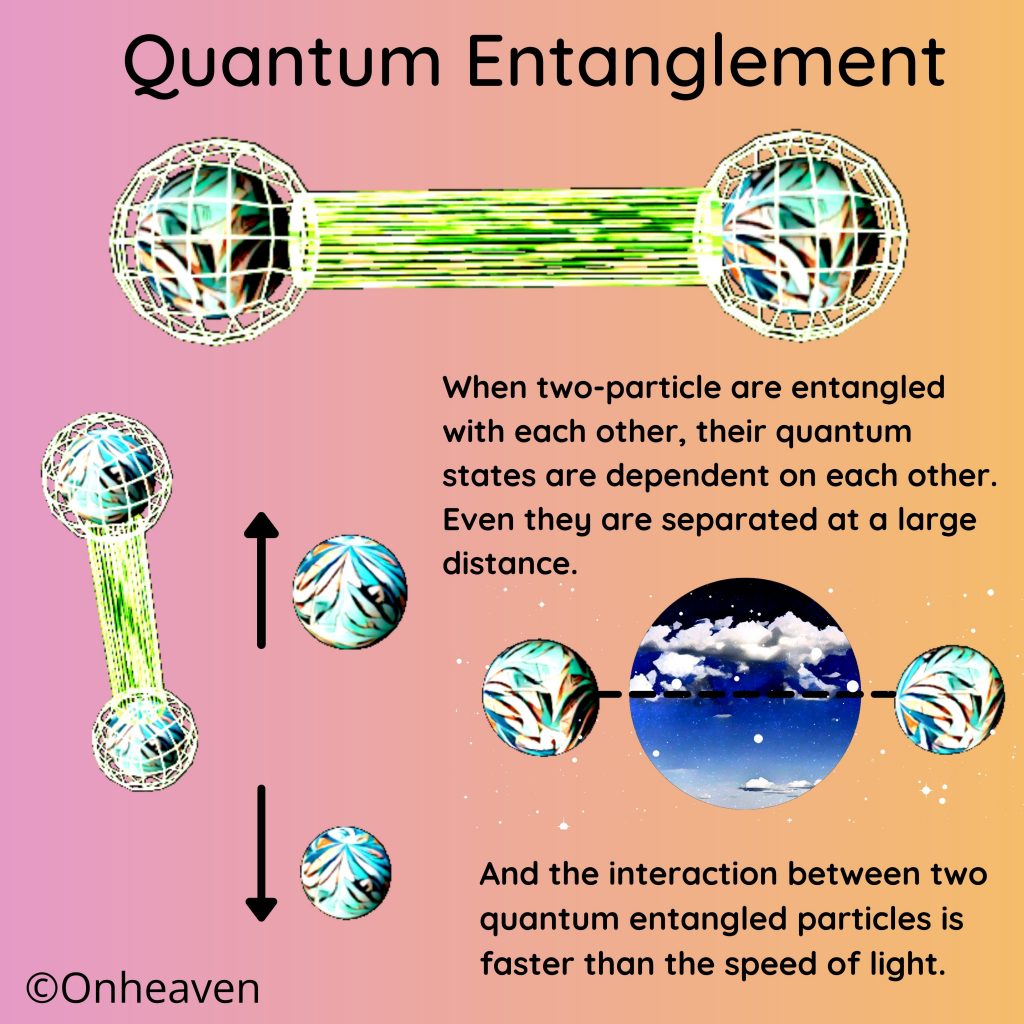
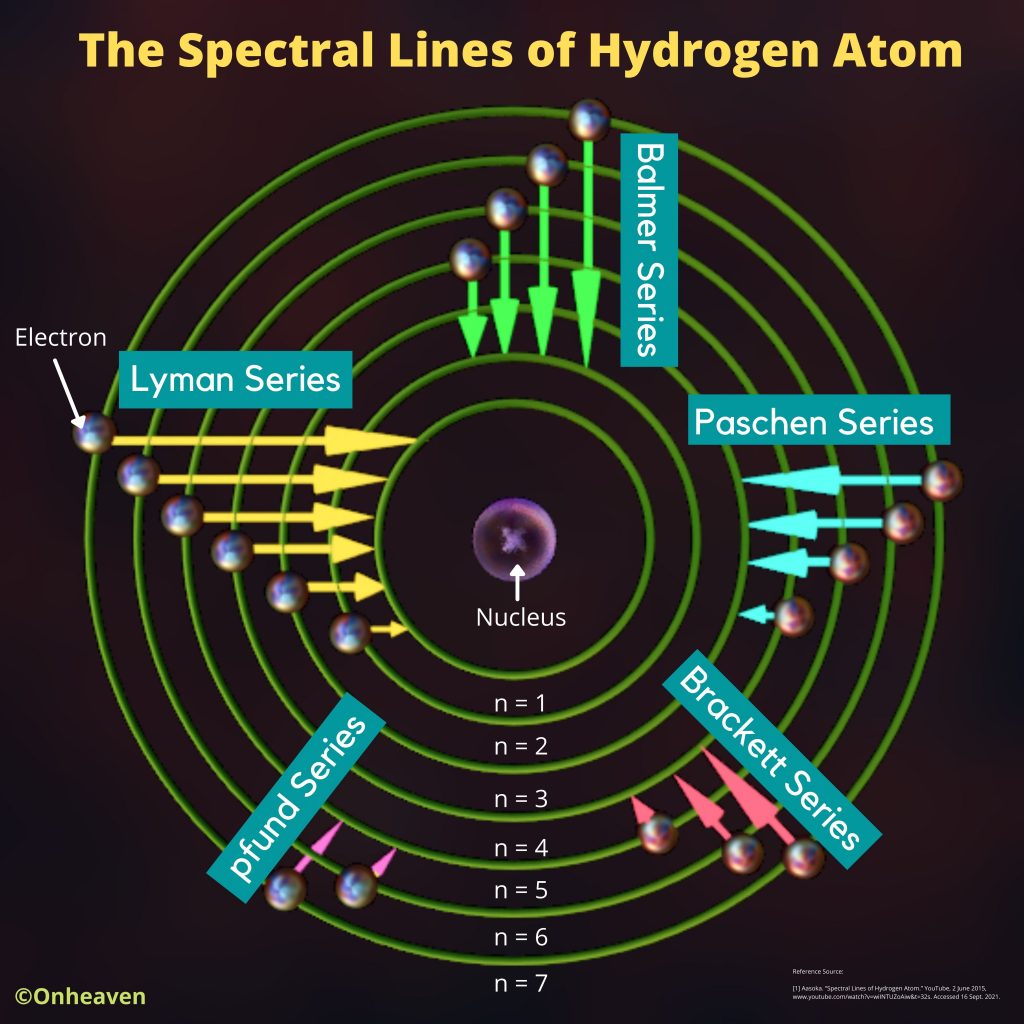















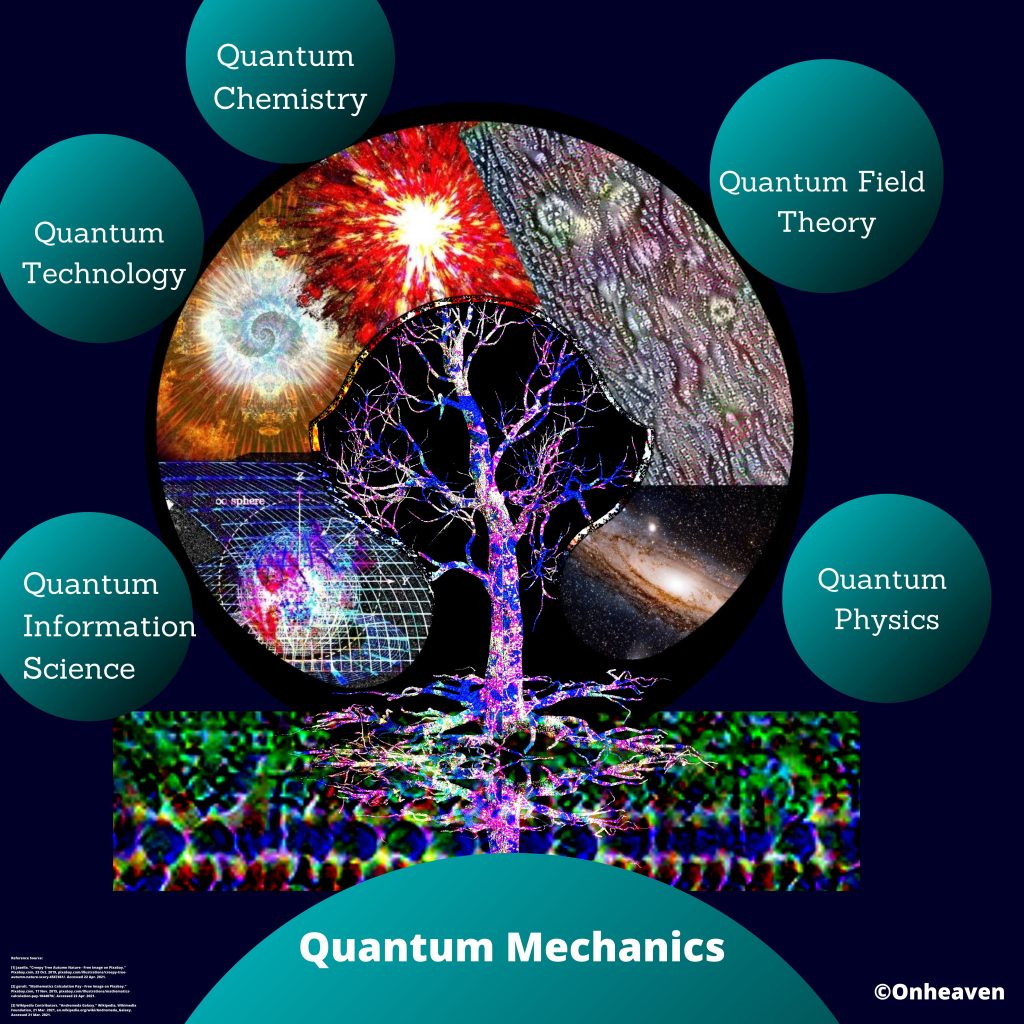
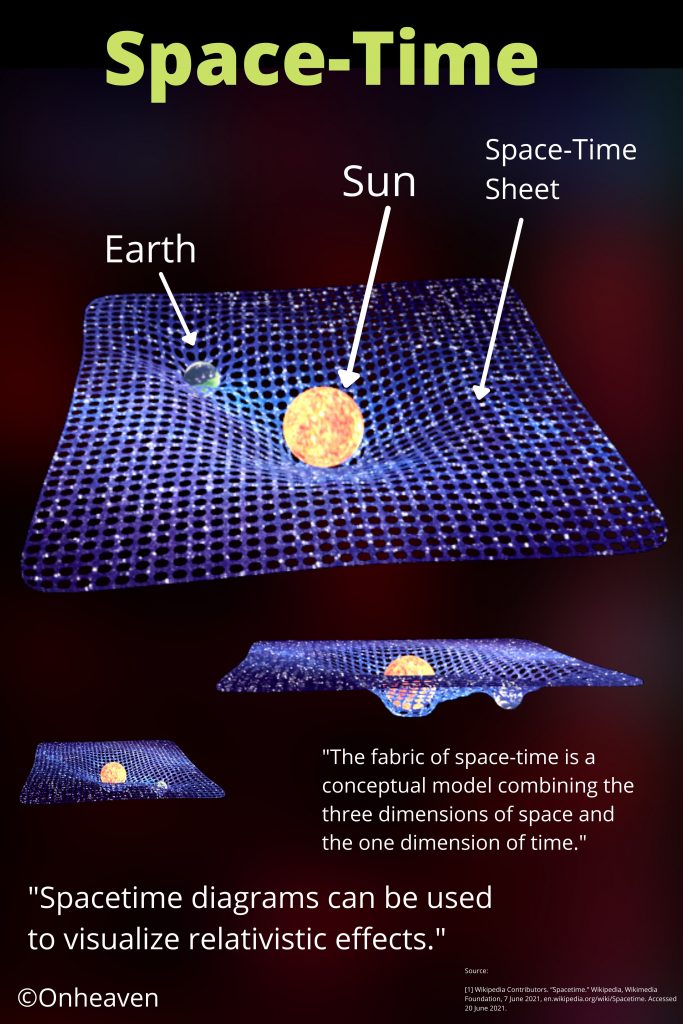
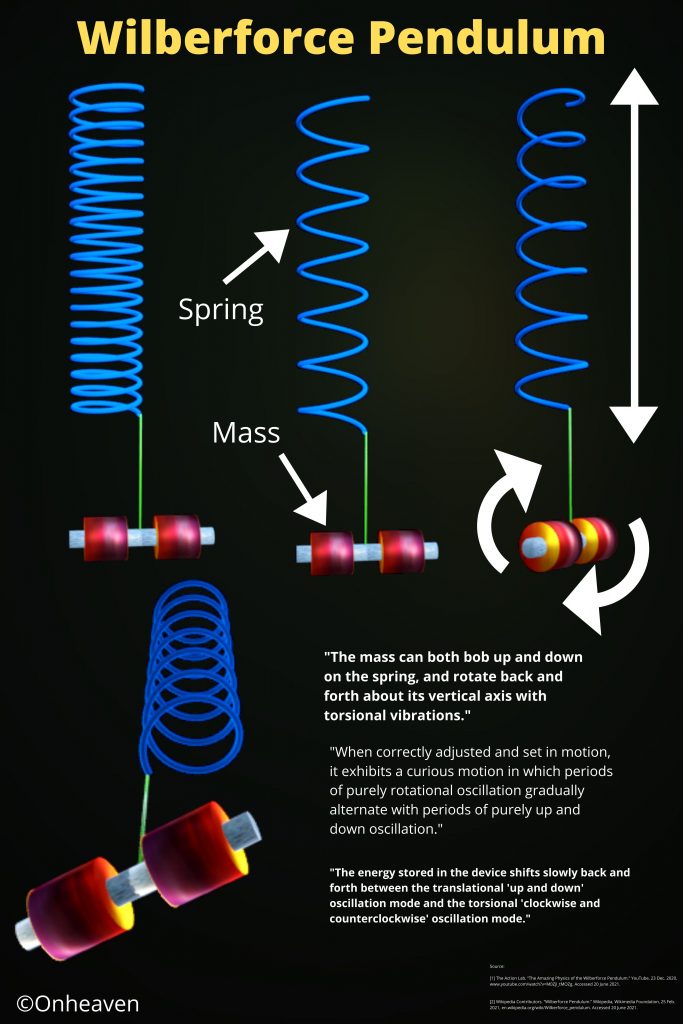
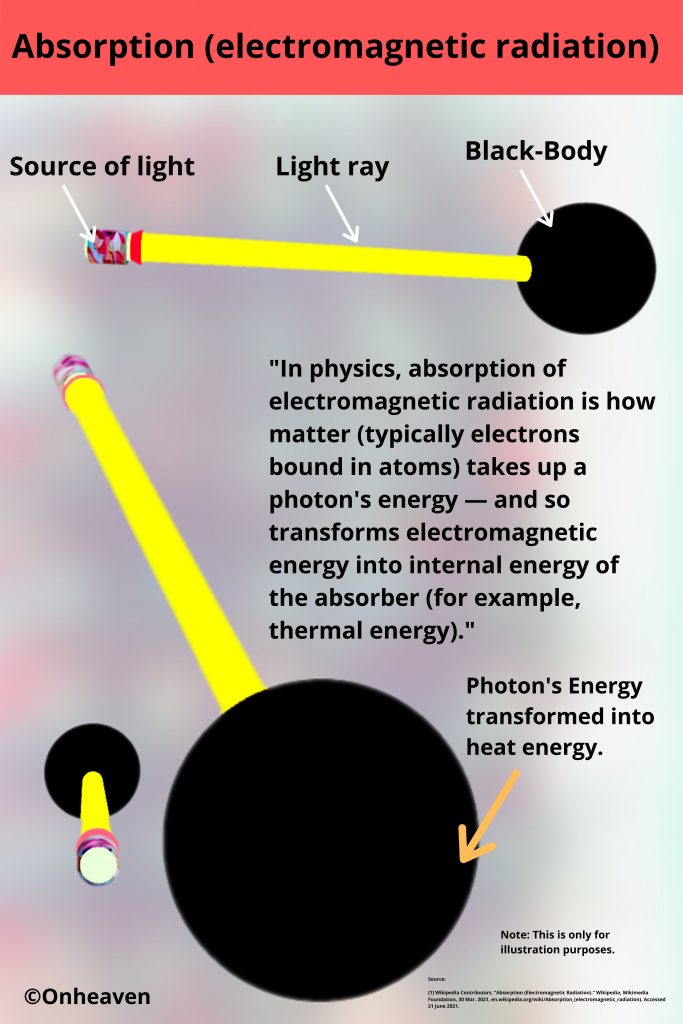


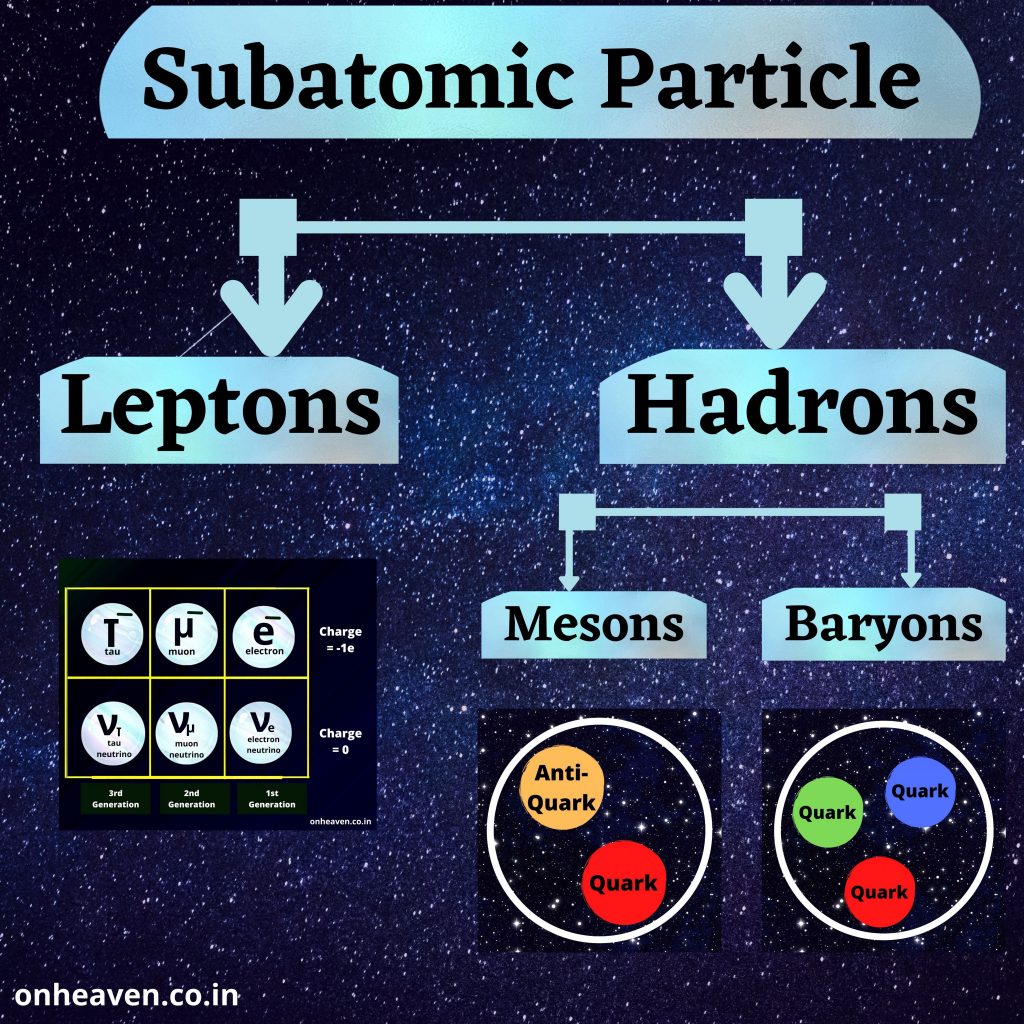
Advertisement
Advertisement

Advertisement
Advertisement

Advertisement

Advertisement

Advertisement
Advertisement

Advertisement

Advertisement
Advertisement
Advertisement
Advertisement
Advertisement
Advertisement
Advertisement



Advertisement

Advertisement

Advertisement


Advertisement


Advertisement


Advertisement

Advertisement