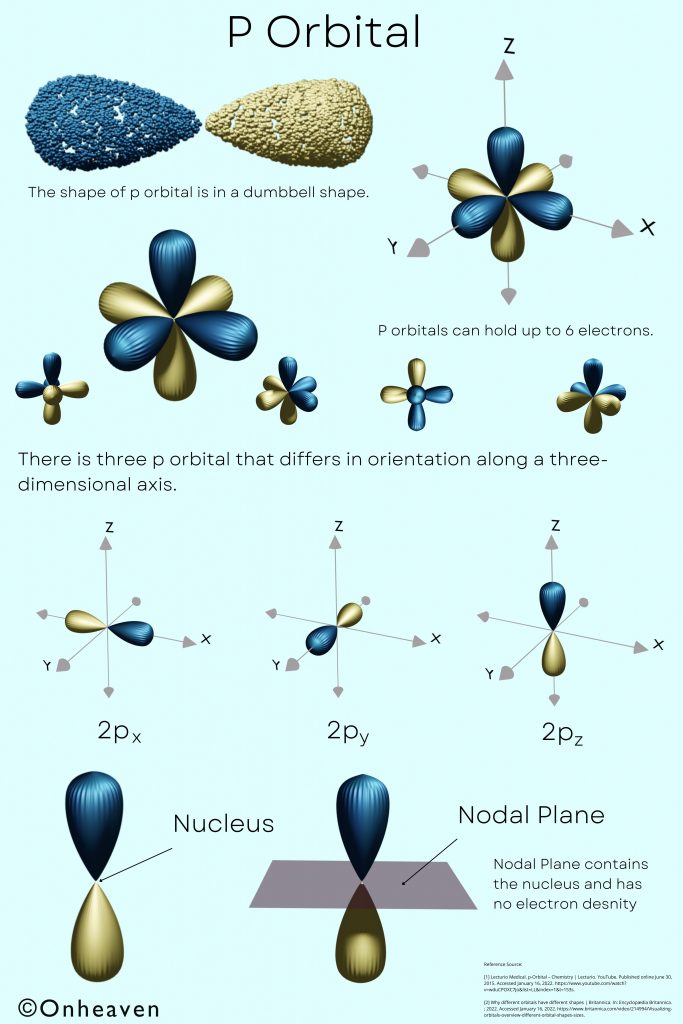
The shape of p orbital is in a dumbbell shape.
There is three p orbital that differs in orientation along a three-dimensional axis.
P orbitals can hold up to 6 electrons.
P orbitals have two regions in which electrons can be found.
Reference Source:
[1] Lecturio Medical. p-Orbital – Chemistry | Lecturio. YouTube. Published online June 30, 2015. Accessed January 16, 2022. https://www.youtube.com/watch?v=wduCPOXC7Jo&list=LL&index=1&t=153s.
[2] Why different orbitals have different shapes | Britannica. In: Encyclopædia Britannica. ; 2022. Accessed January 16, 2022. https://www.britannica.com/video/214994/Visualizing-orbitals-overview-different-orbital-shapes-sizes.