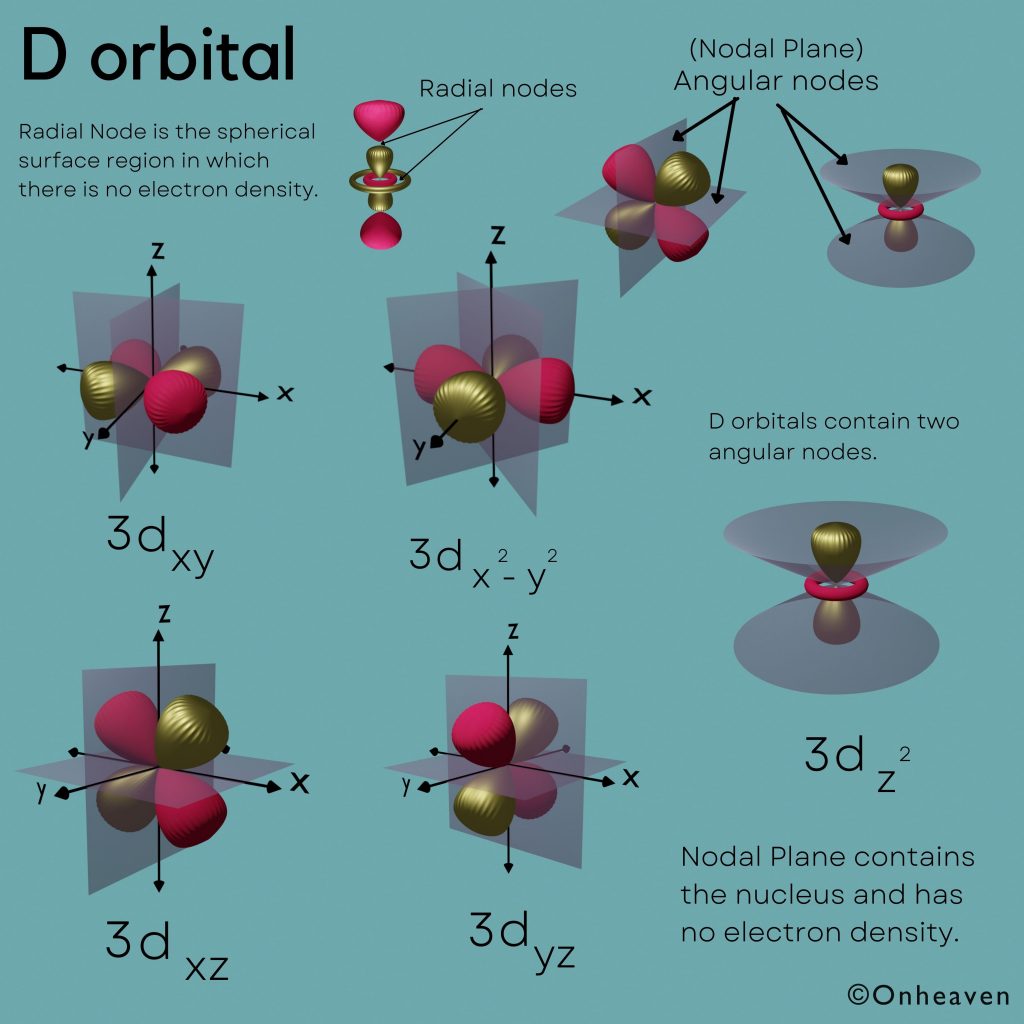
D orbitals contain two angular nodes.
Nodal Plane contains the nucleus and has no electron density.
Radial Node is the spherical surface region in which there is no electron density.
Reference Source:
[1] EduPoint. Shapes of Atomic Orbitals (Part 3) || d-orbital || Chemistry for Class 11 in HINDI. YouTube. Published online September 1, 2019. Accessed January 19, 2022. https://www.youtube.com/watch?v=9R8QNDpkjcM&t=729s.
[2] Radial Nodes. Chemistry LibreTexts. Published January 8, 2017. Accessed January 19, 2022. https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Quantum_Mechanics/09._The_Hydrogen_Atom/Radial_Nodes#:~:text=A%20radial%20node%20is%20a,to%20zero%20or%20changes%20sign%20.
[3] d-orbital | physics | Britannica. In: Encyclopædia Britannica. ; 2022. Accessed January 19, 2022. https://www.britannica.com/science/d-orbital.
[4] How many d orbitals can there be in one energy level? | Socratic. Socratic.org. Published July 20, 2016. Accessed January 19, 2022. https://socratic.org/questions/how-many-d-orbitals-can-there-be-in-one-energy-level-level.
[5] Radial and Angular nodes formula – Definitions, Formula, Calculations, Examples, Diagram , with Videos and FAQ. BYJUS. Published November 25, 2021. Accessed January 19, 2022. https://byjus.com/chemistry/radial-and-angular-nodes-formula/.
[6] d Atomic Orbitals. Chemistry LibreTexts. Published October 2, 2013. Accessed January 19, 2022.https://chem.libretexts.org/Bookshelves/Physical_and_Theoretical_Chemistry_Textbook_Maps/Supplemental_Modules_(Physical_and_Theoretical_Chemistry)/Quantum_Mechanics/09._The_Hydrogen_Atom/Atomic_Orbitals/d_Atomic_Orbitals#:~:text=All%20the%20d%2Dorbitals%20contain,the%20upper%20and%20lower%20lobes.