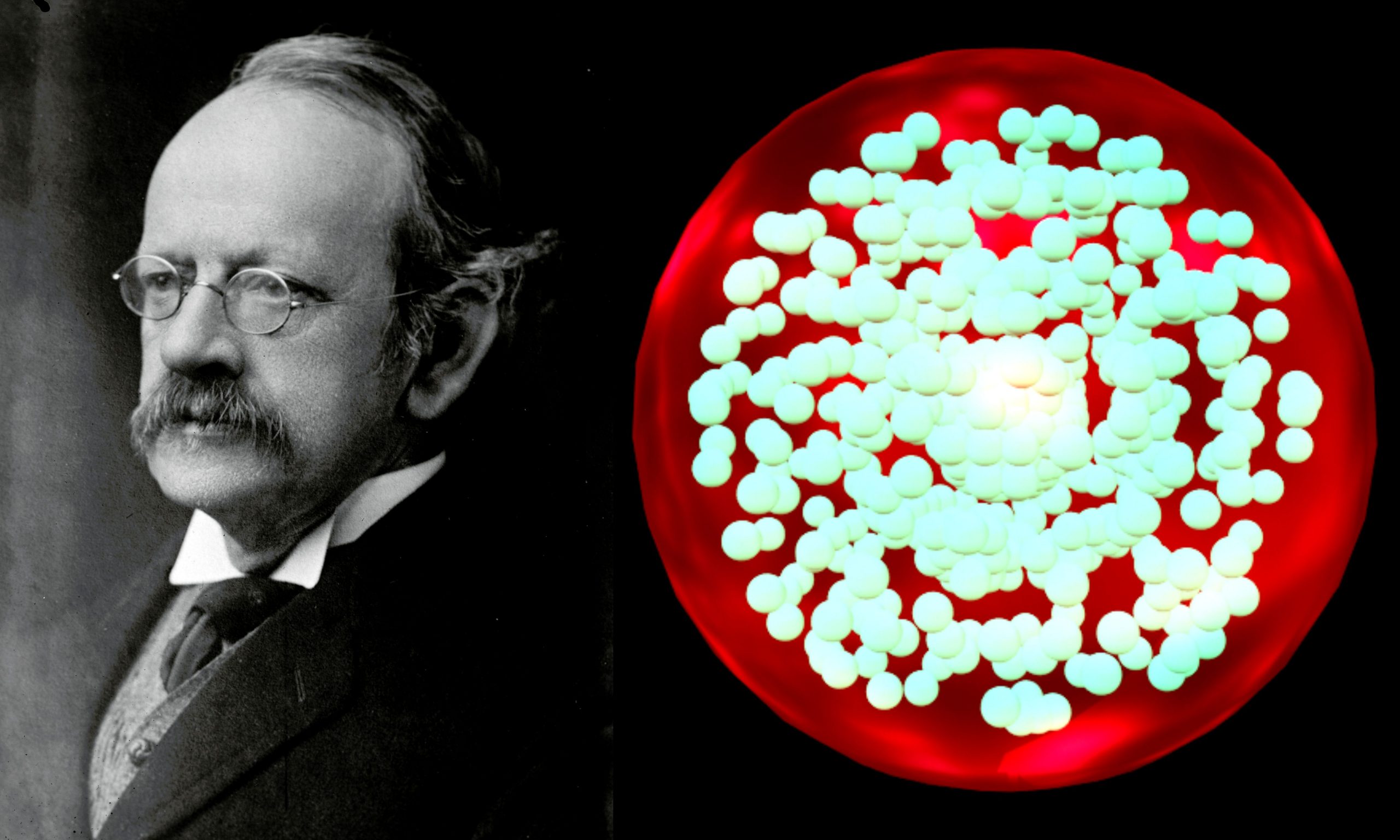
Plum Pudding Model Reference Source: [1] Wikipedia Contributors. “Plum Pudding Model.” Wikipedia, Wikimedia Foundation, 8 July 2021, en.wikipedia.org/wiki/Plum_pudding_model. Accessed 16 Aug. 2021. [2] Wikipedia Contributors.… Read More »The plum pudding model was first proposed by J. J. Thomson in 1904 but before the discovery of the atomic nucleus.
Quantum
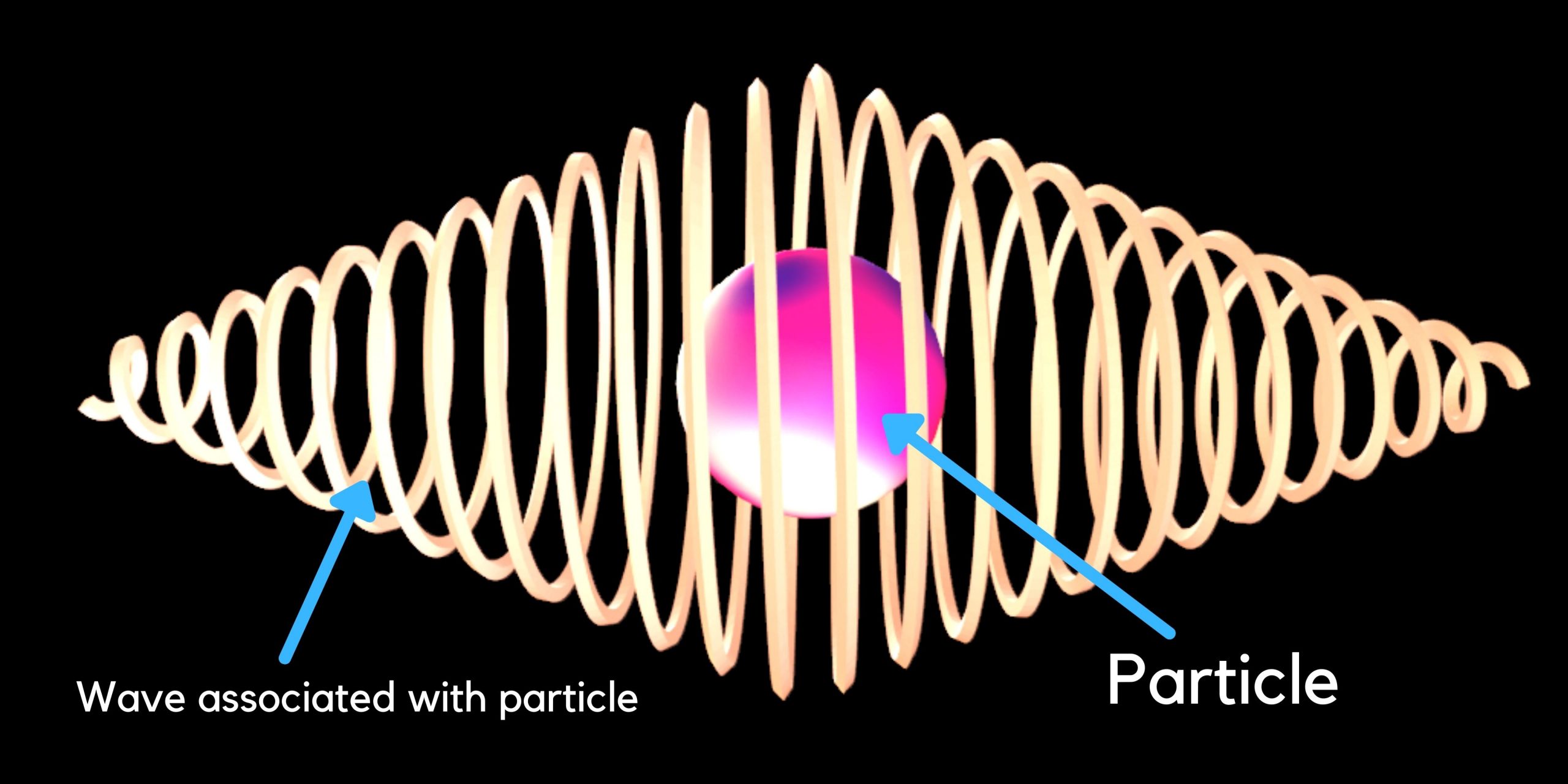
The De Broglie hypothesis proposed that all matter exhibits wave-like behavior which is called a Matter-wave. You may like this book, The Gallery of Quantum… Read More »The De Broglie hypothesis proposed that all matter exhibits wave-like behavior which is called a Matter-wave.
The De Broglie hypothesis proposed that all matter exhibits wave-like behavior which is called a Matter-wave.
Orbital is the space around the nucleus, where there is a high probability of finding electrons. There are 4 types of orbitals: s, p, d,… Read More »Orbital is the space around the nucleus, where there is a high probability of finding electrons.
Orbital is the space around the nucleus, where there is a high probability of finding electrons.
S-Orbital Reference Source: [1] Najam Academy. “The Shapes of Atomic Orbitals S-Orbital, P-Orbital and D-Orbital | #AtomicOrbitals.” YouTube, 15 Mar. 2021, www.youtube.com/watch?v=nNkw_0c8vY0&t=148s. Accessed 22 Sept.… Read More »S-Orbital
S-Orbital
Quantum Entanglement Source: This Illustration on “Quantum Entanglement” is a Part of this Book, The Gallery of Quantum Mechanics The Gallery of Quantum Mechanics
When two-particle are entangled with each other, their quantum states are dependent on each other.
The Spectral Lines of Hydrogen Atom Reference Source: [1] Aasoka. “Spectral Lines of Hydrogen Atom.” YouTube, 2 June 2015, www.youtube.com/watch?v=wiINTUZoAiw&t=32s. Accessed 16 Sept. 2021.
The Spectral Lines of Hydrogen Atom
De Broglie Hypothesis
De Broglie Hypothesis
Wave packet
Wave packet
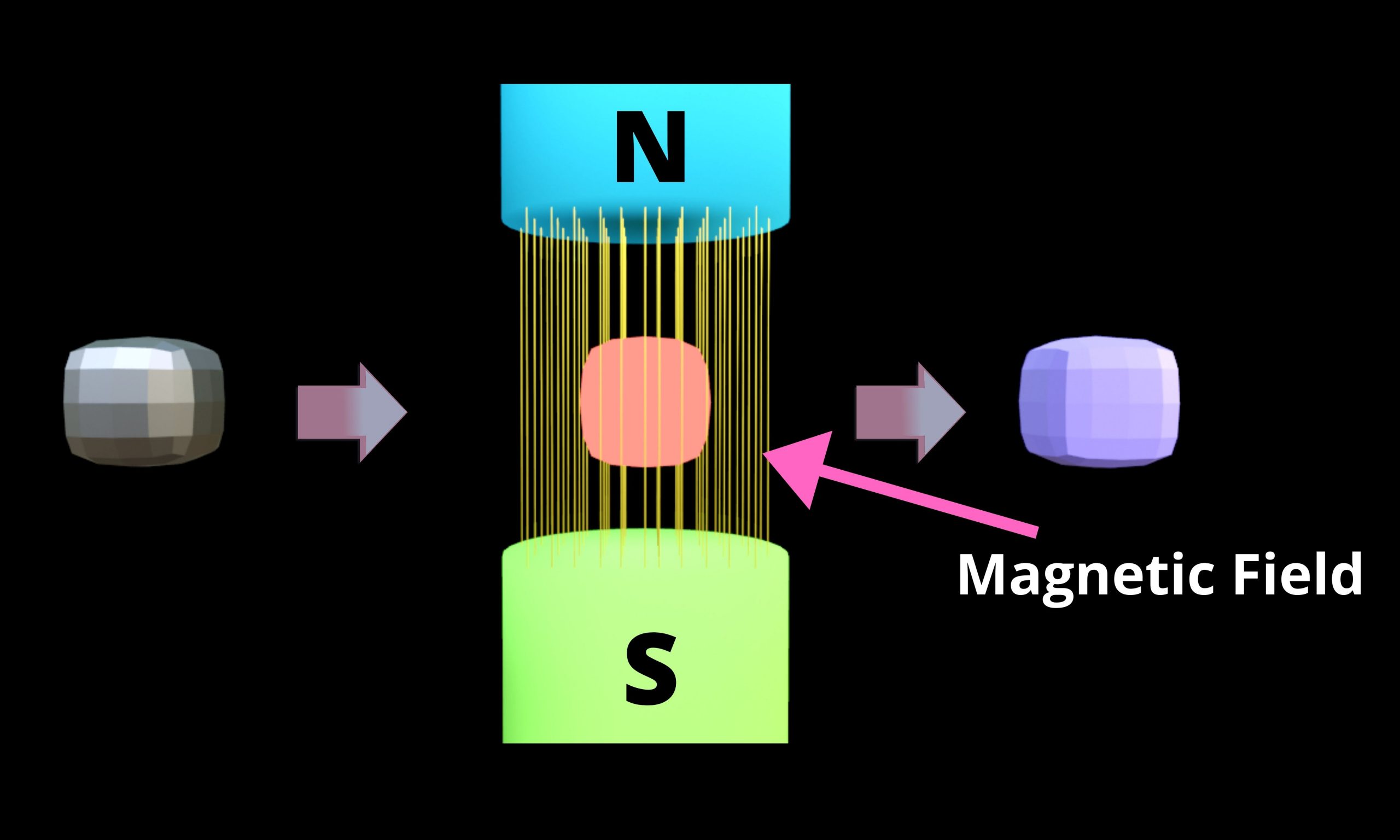
Stern–Gerlach Experiment Reference Source: [1] Wikipedia Contributors. “Stern–Gerlach Experiment.” Wikipedia, Wikimedia Foundation, 12 May 2021, en.wikipedia.org/wiki/Stern-Gerlach_experiment. Accessed 11 Sept. 2021. [2] Butler, David. “Classroom Aid… Read More »Stern–Gerlach Experiment
Stern–Gerlach Experiment
Electric Field due to Circular loop of Charge Reference Source: [1] Eduphile. “Electric Field due to Circular Loop of Charge (Hindi).” YouTube, 9 Sept. 2020,… Read More »Electric Field due to Circular loop of Charge
Electric Field due to Circular loop of Charge
Electric Dipole Moment Reference Source: [1] “A Define Electric Dipole Moment Is It a Scalar or Vector Class 9 Physics CBSE.” Vedantu.com, 16 Sept. 2020,… Read More »Electric Dipole Moment
Electric Dipole Moment
GAUSS’S LAW Carl Friedrich Gauss is regarded as the one of the greatest mathematician of all time.He Published the Gauss’s law in the year 1867.… Read More »GAUSS’S LAW
GAUSS’S LAW
Electromagnetic field is made up of Electric field and Magnetic field This Illustration on “Electromagnetism” is a Part of this Book, The Gallery of Quantum… Read More »Electromagnetic field is made up of Electric field and Magnetic field
Electromagnetic field is made up of Electric field and Magnetic field
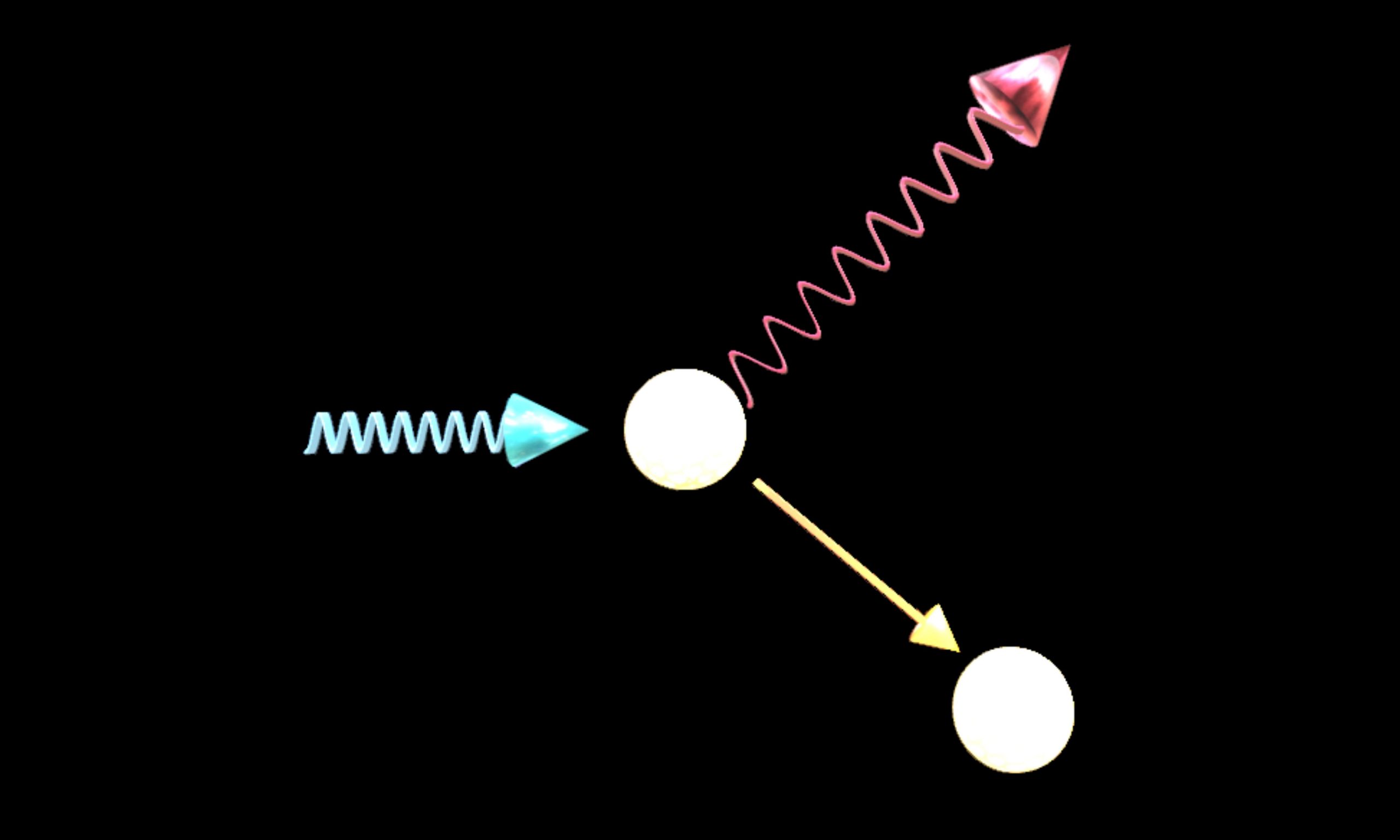
Compton Scattering What is meant by an electron at rest? The electron rest mass is the mass of an electron as measured when its speed is… Read More »Compton Scattering
Compton Scattering
Magnetic refrigeration is a cooling technology based on the magnetocaloric effect. This Illustration on “Magnetic Refrigeration” is a Part of this Book, The Gallery of… Read More »Magnetic refrigeration is a cooling technology based on the magnetocaloric effect.
Magnetic refrigeration is a cooling technology based on the magnetocaloric effect.
The Meissner Effect is the expulsion of a magnetic field from a superconductor. This Illustration on “Meissner effect” is a Part of this Book, The… Read More »The Meissner Effect is the expulsion of a magnetic field from a superconductor.
The Meissner Effect is the expulsion of a magnetic field from a superconductor.
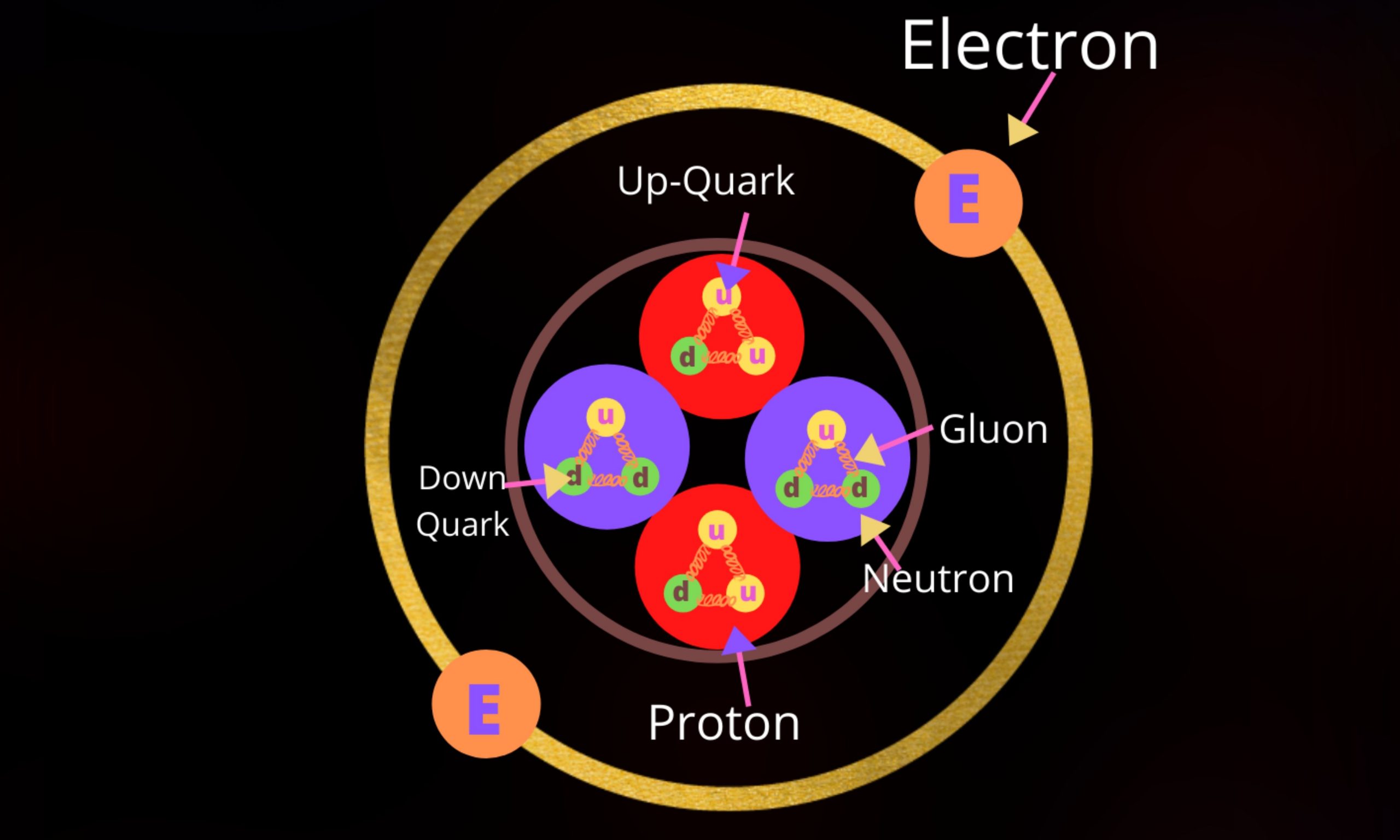
Quark Model of an Atom This Illustration on “Quark Model of an Atom” is a Part of this Book, The Gallery of Quantum Mechanics The… Read More »Quark Model of an Atom
Quark Model of an Atom
Hawking Radiation This Illustration on “Hawking Radiation” is a Part of this Book, The Gallery of Quantum Mechanics The Gallery of Quantum Mechanics
What is Hawking Radiation? Explanation with Illustration Diagrams.
Rutherford Alpha Particle Scattering Experiment This illustration on “Rutherford Alpha Particle Scattering Experiment” is a Part of this Book, QUANTUM PHYSICS for Beginners. QUANTUM PHYSICS… Read More »Rutherford Alpha Particle Scattering Experiment
Rutherford Alpha Particle Scattering Experiment
Helium-Neon Laser
Simple Cubic Crystal Structure
Helium-Neon Laser Reference Source: [1] Wikipedia Contributors. “Helium–Neon Laser.” Wikipedia, Wikimedia Foundation, 2 May 2021, en.wikipedia.org/wiki/Helium%E2%80%93neon_laser. Accessed 31 Aug. 2021. [2] Eduphile. “He Ne Laser… Read More »Helium-Neon Laser
Helium-Neon Laser
Ruby Laser Reference Source: [1] Wikipedia Contributors. “Ruby Laser.” Wikipedia, Wikimedia Foundation, 13 July 2021, en.wikipedia.org/wiki/Ruby_laser. Accessed 30 Aug. 2021.
Ruby Laser
Total Internal Reflection Reference Source: [1] Khan Academy. “Total Internal Reflection | Geometric Optics | Physics | Khan Academy.” YouTube, 9 Dec. 2010, www.youtube.com/watch?v=WRuatAcd2WY. Accessed… Read More »Total Internal Reflection
Total Internal Reflection
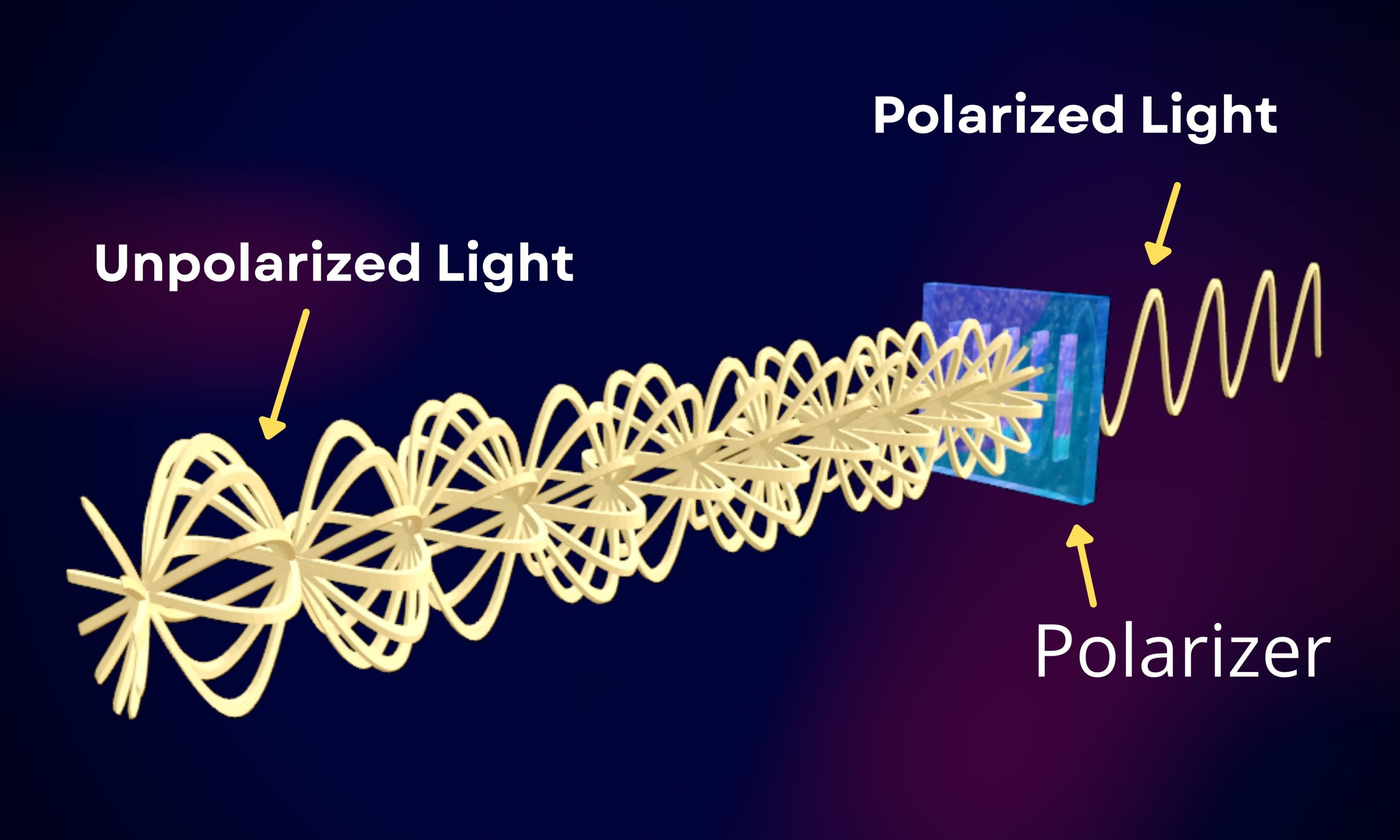
Polarization of Light Reference Source: [1] “Polarization of Light | Olympus LS.” Olympus-Lifescience.com, 2020, www.olympus-lifescience.com/en/microscope-resource/primer/lightandcolor/polarization/. Accessed 29 Aug. 2021. [2] Wikipedia Contributors. “Polarizer.” Wikipedia, Wikimedia… Read More »Polarization of Light
Polarization of Light
Polarization of Light Reference Source: [1] “Polarization of Light | Olympus LS.” Olympus-Lifescience.com, 2020, www.olympus-lifescience.com/en/microscope-resource/primer/lightandcolor/polarization/. Accessed 29 Aug. 2021. [2] Wikipedia Contributors. “Polarizer.” Wikipedia, Wikimedia… Read More »Polarization of Light
Polarization of Light
Quantum dots are Nano-size man-made crystals that have the ability to convert a spectrum of light into different colors. This Illustration on “Quantum Dot” is… Read More »Quantum dots are Nano-size man-made crystals that have the ability to convert a spectrum of light into different colors.
Quantum dots are Nano-size man-made crystals that have the ability to convert a spectrum of light into different colors.
Stimulated Absorption
Stimulated Absorption
Stimulated Emission
Stimulated Emission
If Light Has No Mass, then why it is Affected by Black Holes This Illustration on “If Light Has No Mass, then why it is… Read More »If Light Has No Mass, then why it is Affected by Black Holes
If Light Has No Mass, then why it is Affected by Black Holes
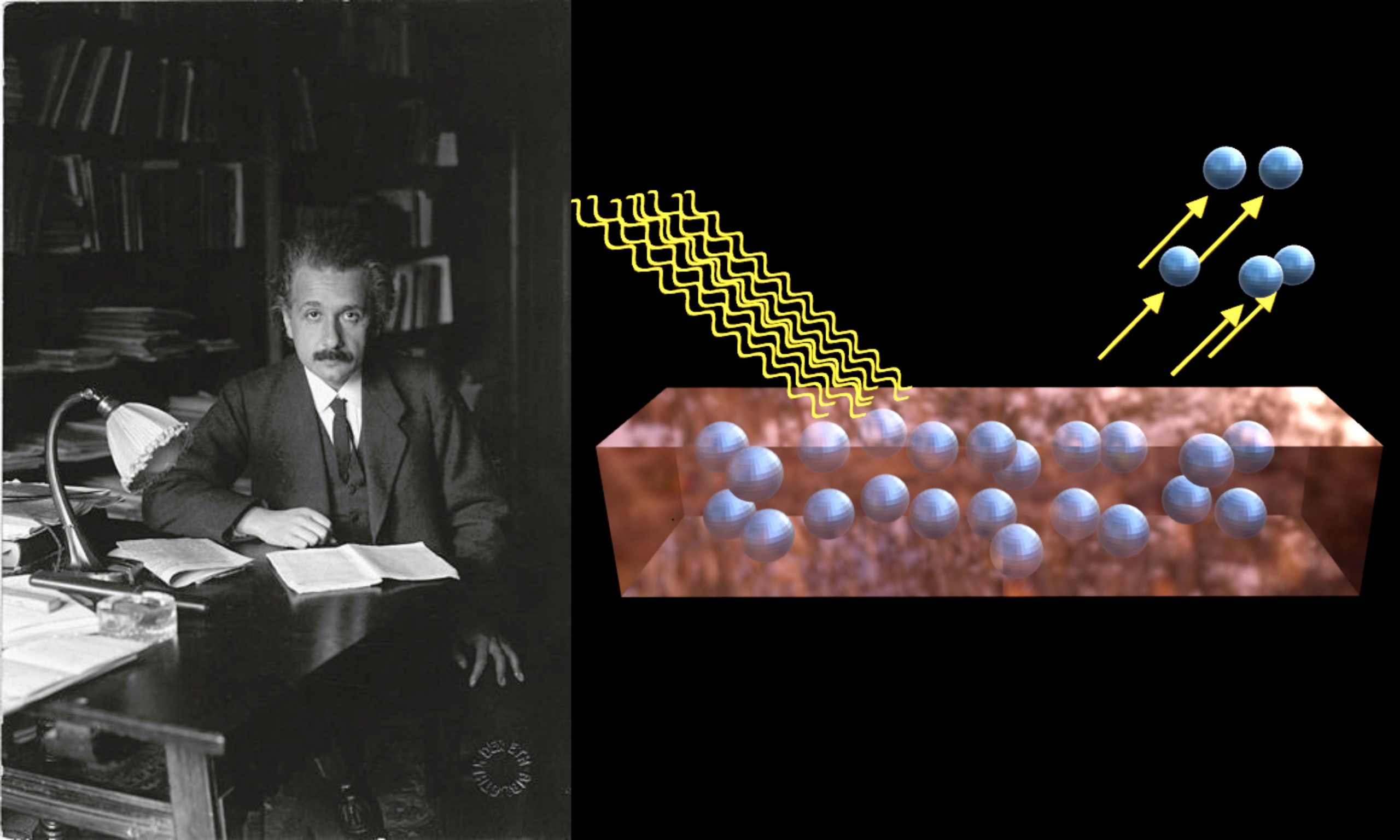
Photoelectric Effect Reference Source: [1] Wikipedia Contributors. “Photoelectric Effect.” Wikipedia, Wikimedia Foundation, 11 Aug. 2021, en.wikipedia.org/wiki/Photoelectric_effect. Accessed 13 Aug. 2021. [2] Wikipedia Contributors. “Albert Einstein.”… Read More »Photoelectric Effect
Photoelectric Effect
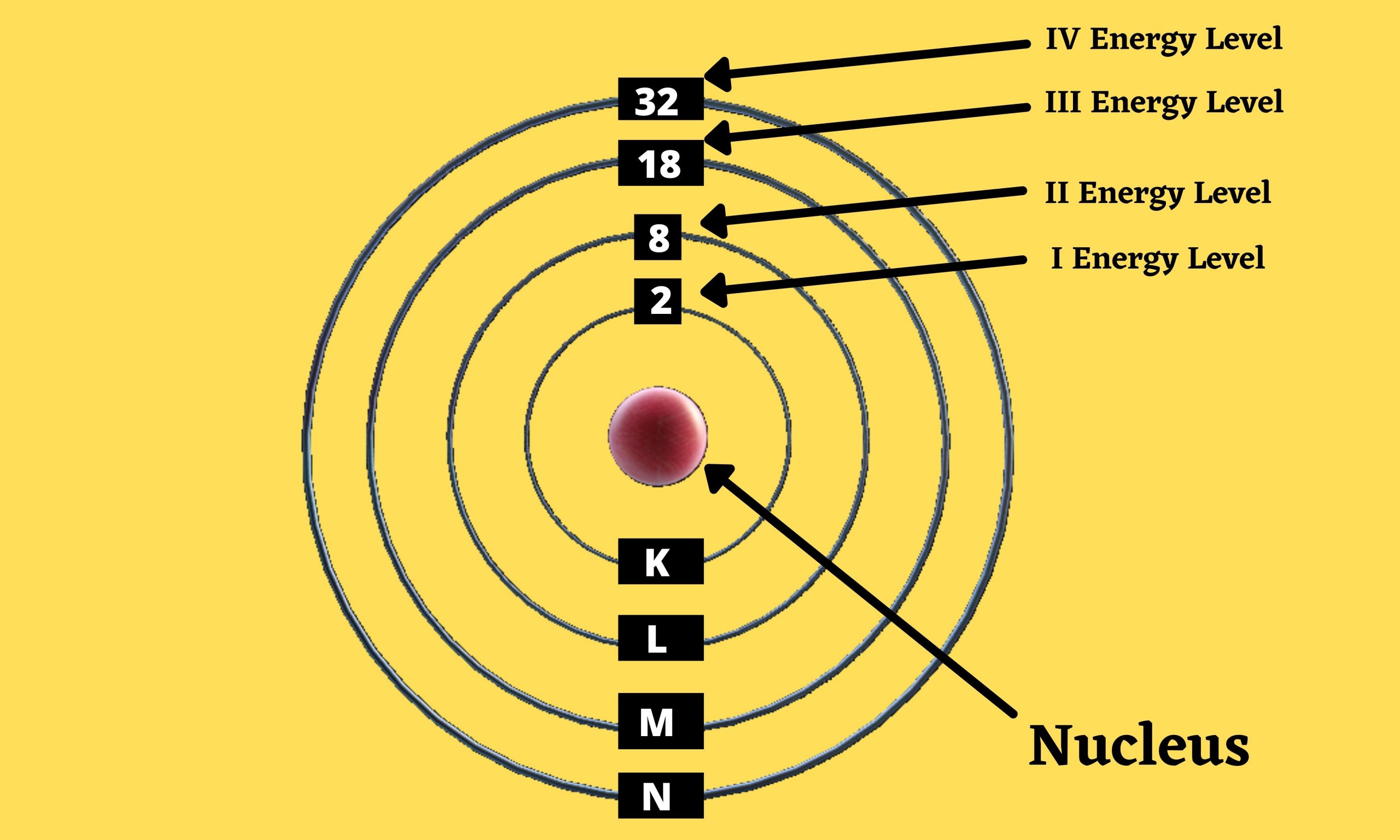
Quantum Mechanics: Atomic Energy Level K,L,M,N notation is use for showing the no. of electron in an orbits of different shells. After absorbing energy, an… Read More »Quantum Mechanics: Atomic Energy Level
Quantum Mechanics: Atomic Energy Level
Crystal Structure: Body-centered Cubic Unit Cell (BCC) You may find this book interesting, “Experience an illustration based learning” CLICK HERE TO KNOW MORE
Crystal Structure: Body-centered Cubic Unit Cell (BCC)
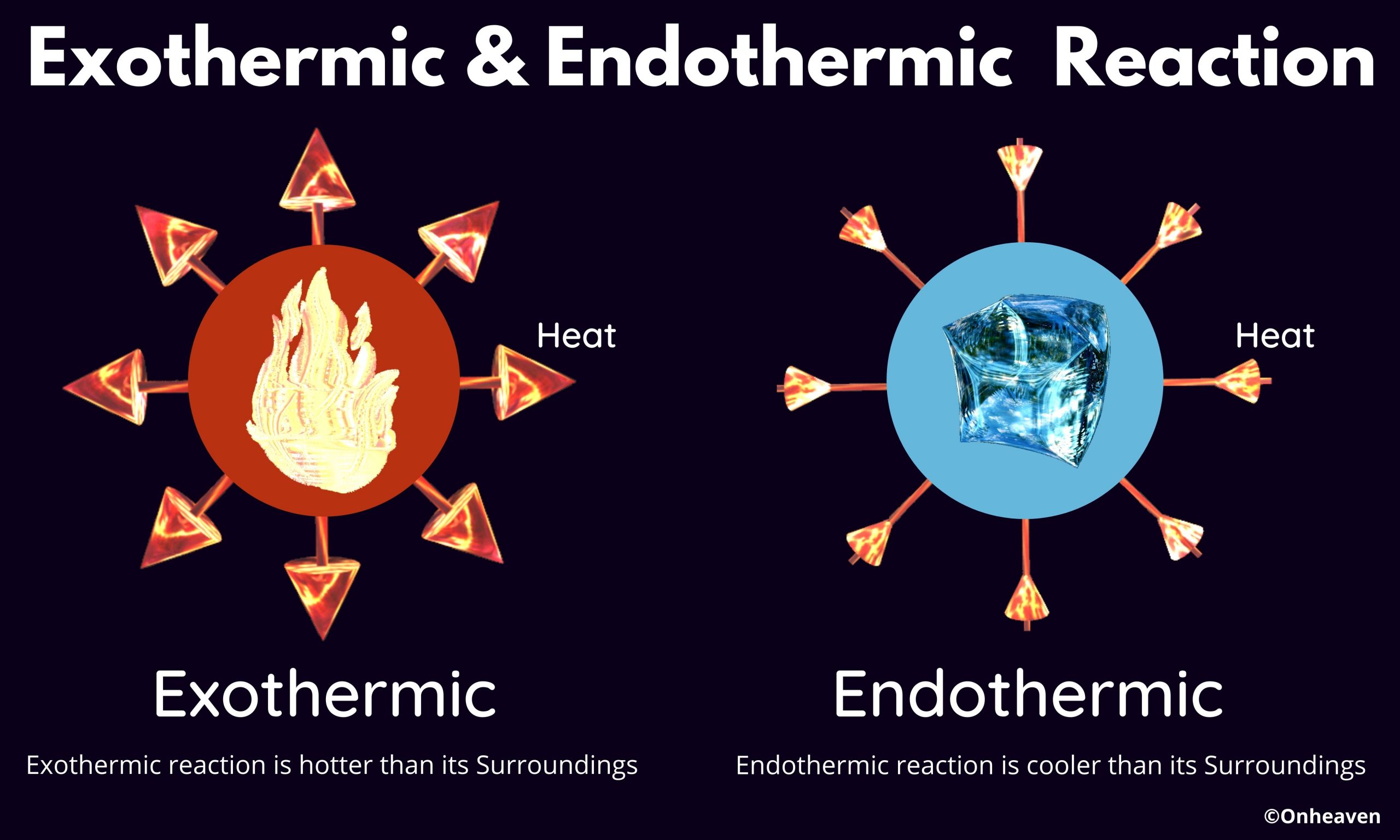
Exothermic & Endothermic Reaction You may find this book interesting, “Experience an illustration based learning” CLICK HERE TO KNOW MORE
Exothermic & Endothermic Reaction
Discovery of Electron Diffraction Sir George Paget Thomson: Physicist and Nobel laureate in Physics. Recognized for his discovery of the wave properties of the electron… Read More »Discovery of Electron Diffraction
Discovery of Electron Diffraction
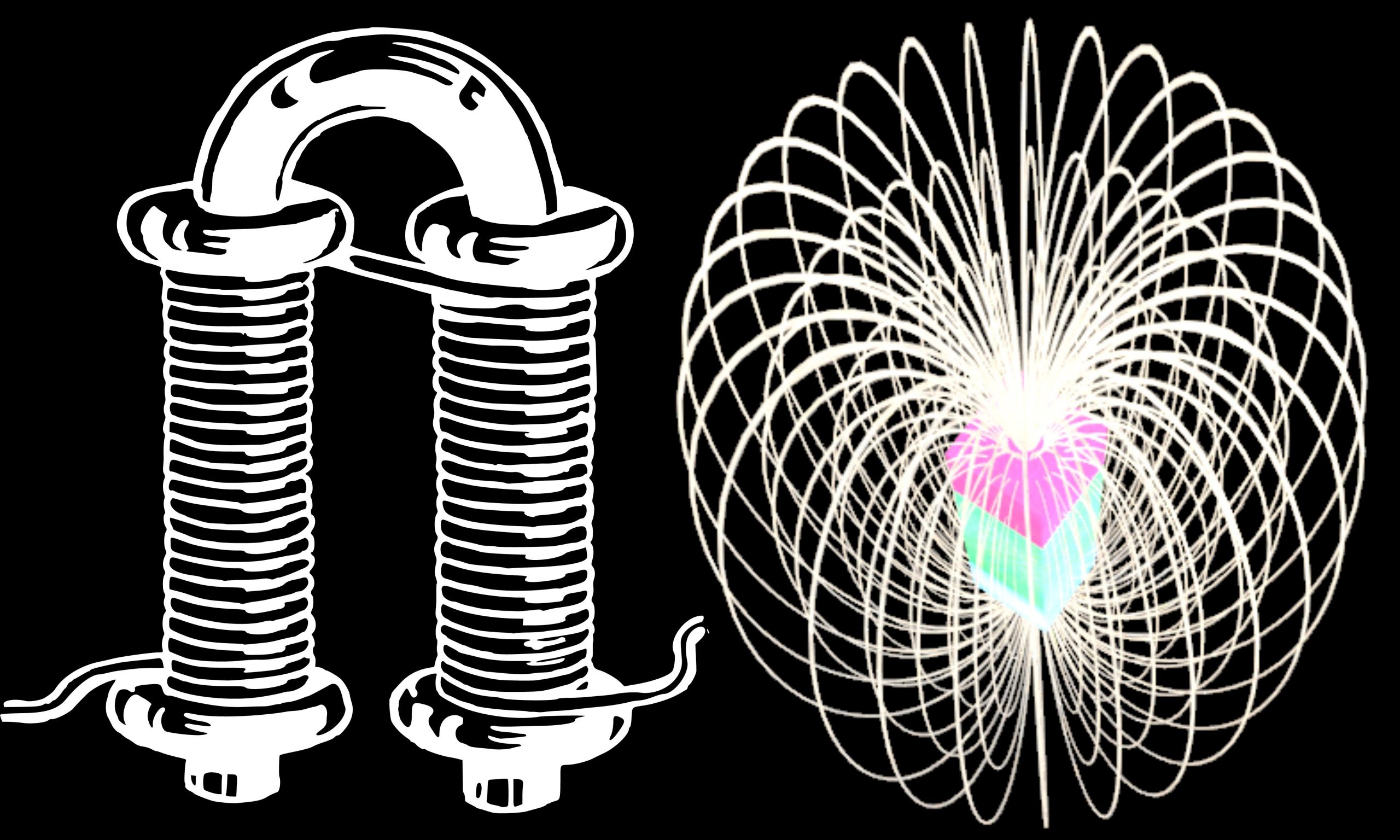
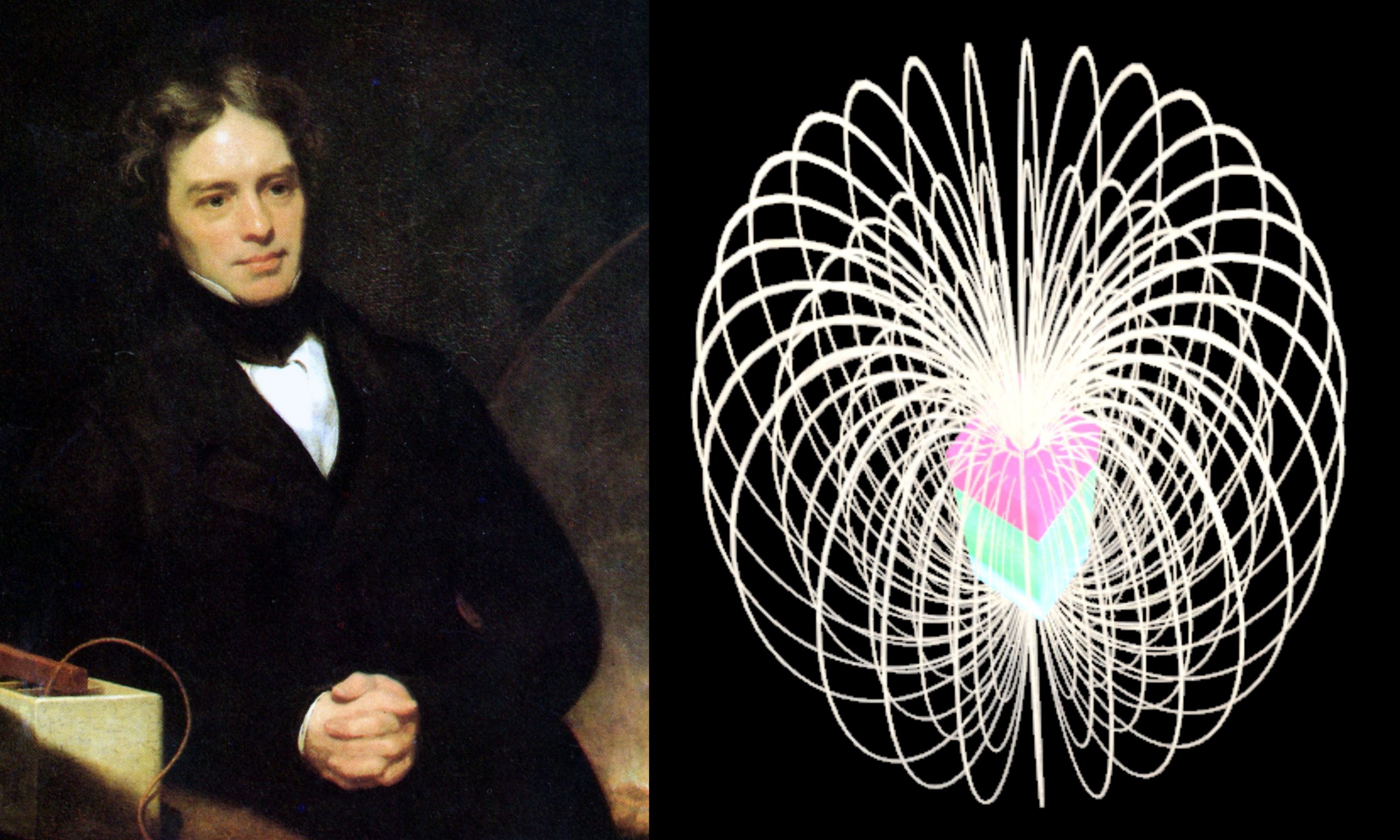
Faraday’s Law of Induction Reference Source: [1] Wikipedia Contributors. “Michael Faraday.” Wikipedia, Wikimedia Foundation, 8 Aug. 2021, en.wikipedia.org/wiki/Michael_Faraday. Accessed 21 Aug. 2021. [2] Wikipedia Contributors.… Read More »Faraday’s Law of Induction
Faraday’s Law of Induction
Interference Diagram of Wave: “The principle of superposition of waves states that when two or more propagating waves of the same type are incident on… Read More »Quantum Mechanics: Interference Diagram of Wave
Quantum Mechanics: Interference Diagram of Wave
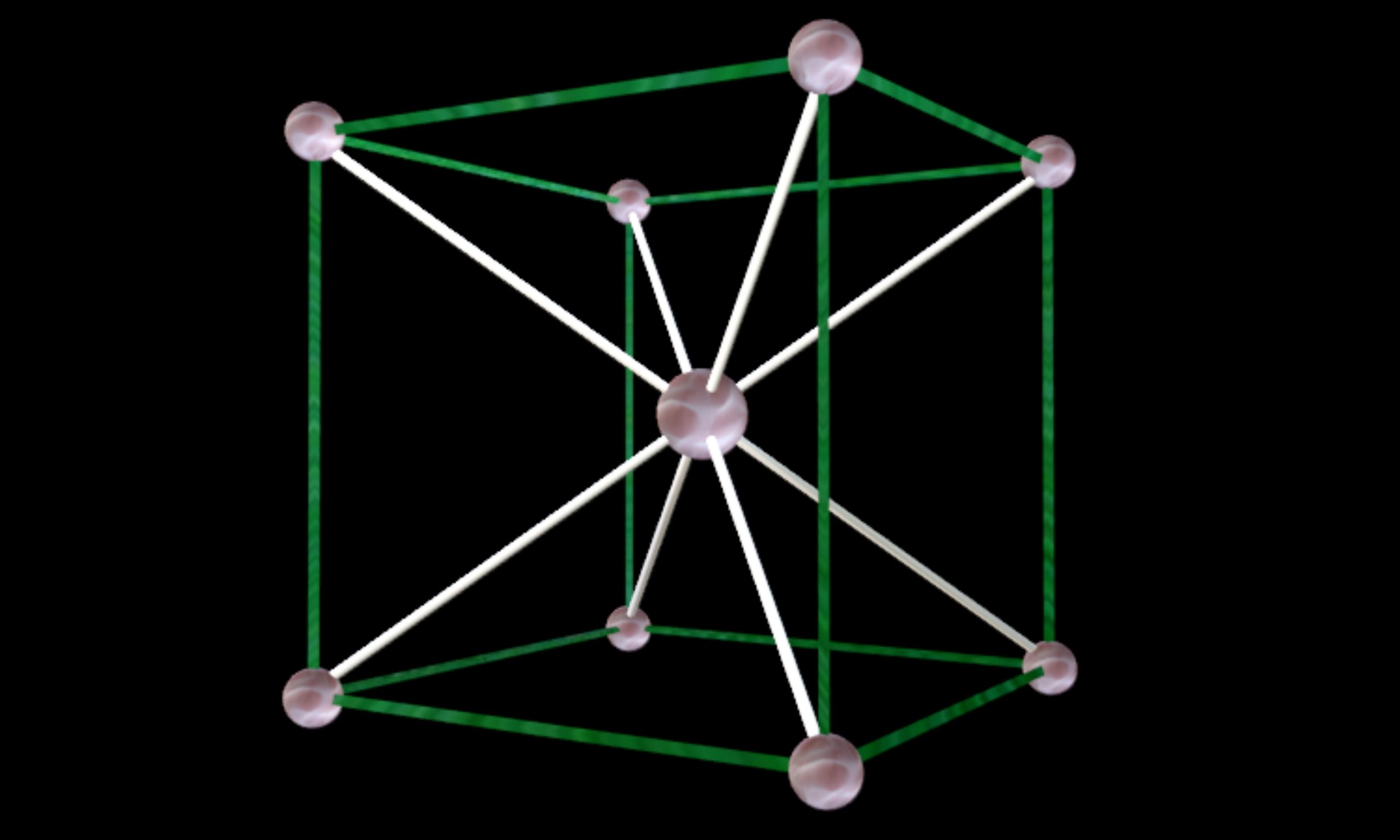
Body-Centered Cubic Unit Cell
Body-Centered Cubic Unit Cell
Interferometer: What is Interferometry: Interferometry is a technique in which waves are superimposed to cause the phenomenon of interference. The technique is used in the… Read More »Quantum Mechanics: Interferometer
Quantum Mechanics: Interferometer
In 1922, the Nobel Prize in Physics is awarded to Neils Bohr for his contribution to the “investigation of the structure of atoms and the… Read More »In 1922, the Nobel Prize in Physics is awarded to Neils Bohr for his contribution to the “investigation of the structure of atoms and the radiation emanating from them.”
In 1922, the Nobel Prize in Physics is awarded to Neils Bohr for his contribution to the “investigation of the structure of atoms and the radiation emanating from them.”
Davisson–Germer Experiment: “The Davisson–Germer experiment was a 1923-27 experiment. Electrons scattered by the surface of a crystal of nickel metal displayed a diffraction pattern. This… Read More »Quantum Mechanics: Davisson–Germer Experiment
Quantum Mechanics: Davisson–Germer Experiment
Discovery of the Wave Nature of the Electron Reference Source: [1] “The Nobel Prize in Physics 1929.” NobelPrize.org, 2021, www.nobelprize.org/prizes/physics/1929/broglie/facts/. Accessed 17 Aug. 2021. “Experience… Read More »Discovery of the Wave Nature of the Electron
Discovery of the Wave Nature of the Electron
Plum Pudding Model Reference Source: [1] Wikipedia Contributors. “Plum Pudding Model.” Wikipedia, Wikimedia Foundation, 8 July 2021, en.wikipedia.org/wiki/Plum_pudding_model. Accessed 16 Aug. 2021. [2] Wikipedia Contributors.… Read More »The plum pudding model was first proposed by J. J. Thomson in 1904 but before the discovery of the atomic nucleus.
The plum pudding model was first proposed by J. J. Thomson in 1904 but before the discovery of the atomic nucleus.
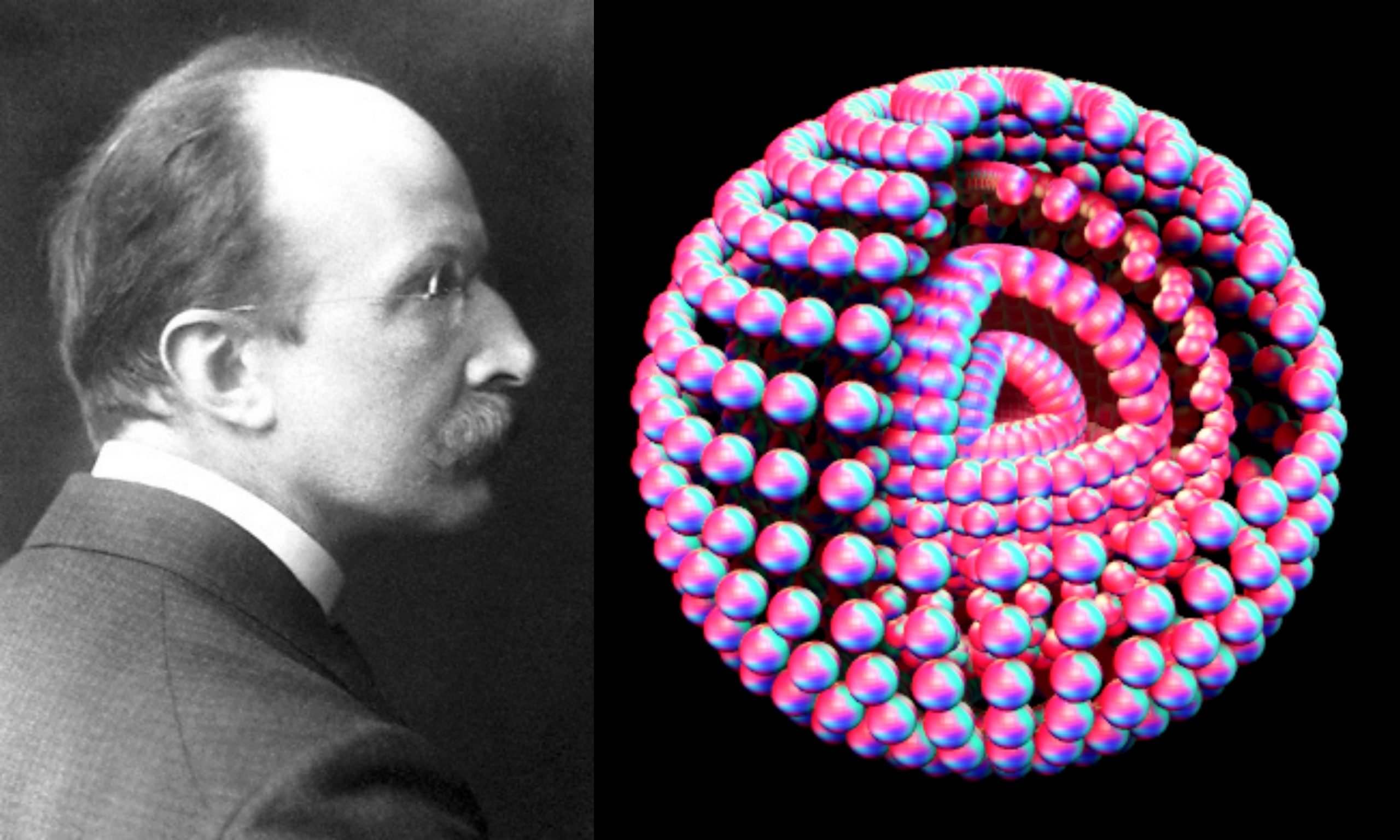
In 1918, Max Planck was awarded the Nobel Prize in Physics for the Discovery of Energy Quanta. Reference Source: [1] Wikipedia Contributors. “Max Planck.” Wikipedia,… Read More »In 1918, Max Planck was awarded the Nobel Prize in Physics for the Discovery of Energy Quanta.
In 1918, Max Planck was awarded the Nobel Prize in Physics for the Discovery of Energy Quanta.
By clicking on the buy button you will be redirected to Amazon.
The Gallery of Quantum Mechanics:
Photoelectric Effect This Illustration on “Photoelectric Effect” is a Part of this Book, The Gallery of Quantum Mechanics The Gallery of Quantum Mechanics Reference Source:… Read More »“In 1905, Einstein proposed a theory of the photoelectric effect.”
“In 1905, Einstein proposed a theory of the photoelectric effect.”
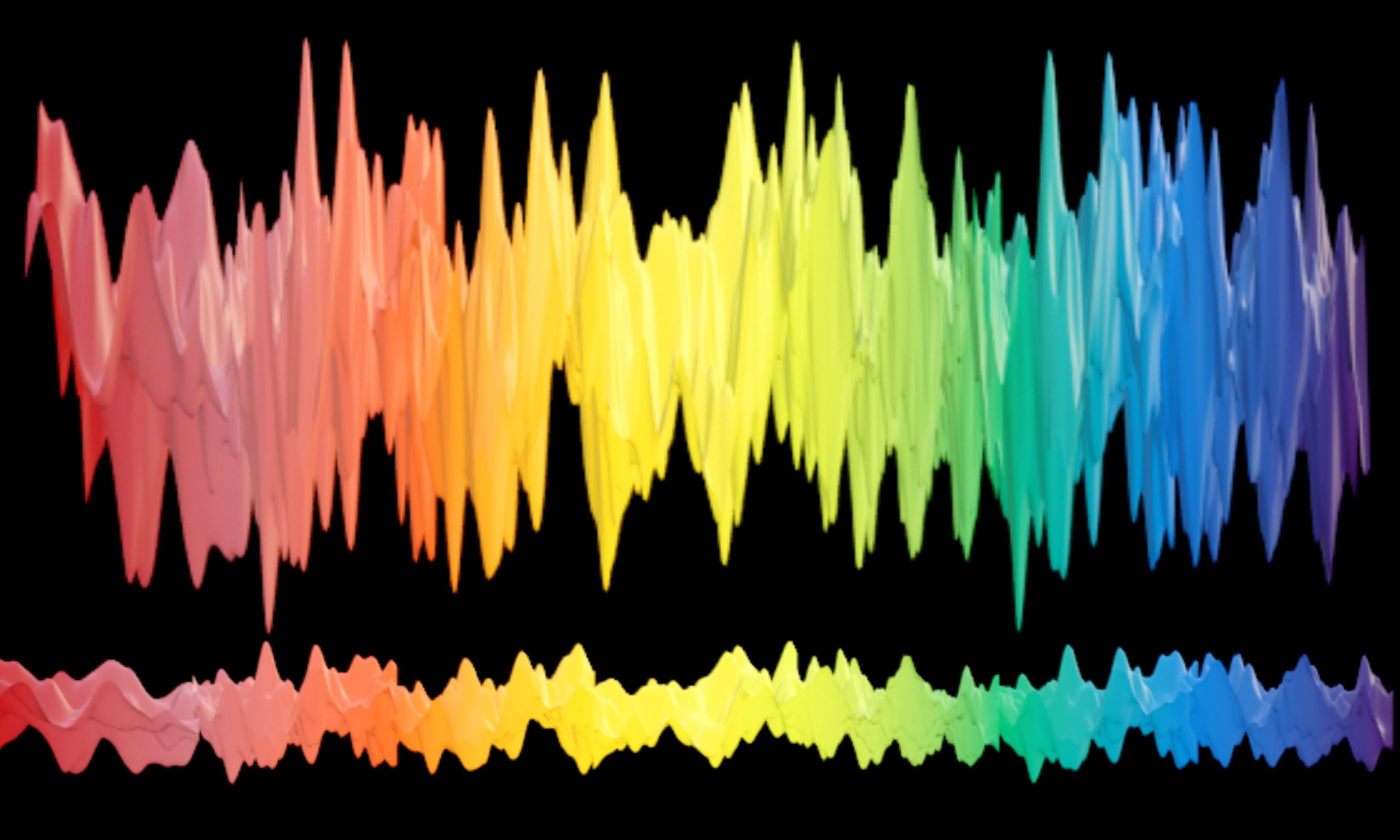
Visible Light Spectrum
Visible Light Spectrum
Energy Band Diagram of Semi-Conductor: You may find this book interesting, “Experience an illustration based learning” CLICK HERE TO KNOW MORE
Energy Band Diagram of Semi-Conductor
Layers of the Atmosphere:
Layers of the Atmosphere
Double-Slit Experiment: “The experiment was first performed, using light, by Thomas Young in 1801, as a demonstration of the wave behavior of light.” “In 1927,… Read More »Double-Slit Experiment
Double-Slit Experiment
Discovery of Positron: Reference Source: [1] Wikipedia Contributors. “Carl David Anderson.” Wikipedia, Wikimedia Foundation, 8 Aug. 2021, en.wikipedia.org/wiki/Carl_David_Anderson. Accessed 10 Aug. 2021. [2] Wikipedia Contributors.… Read More »Discovery of Positron