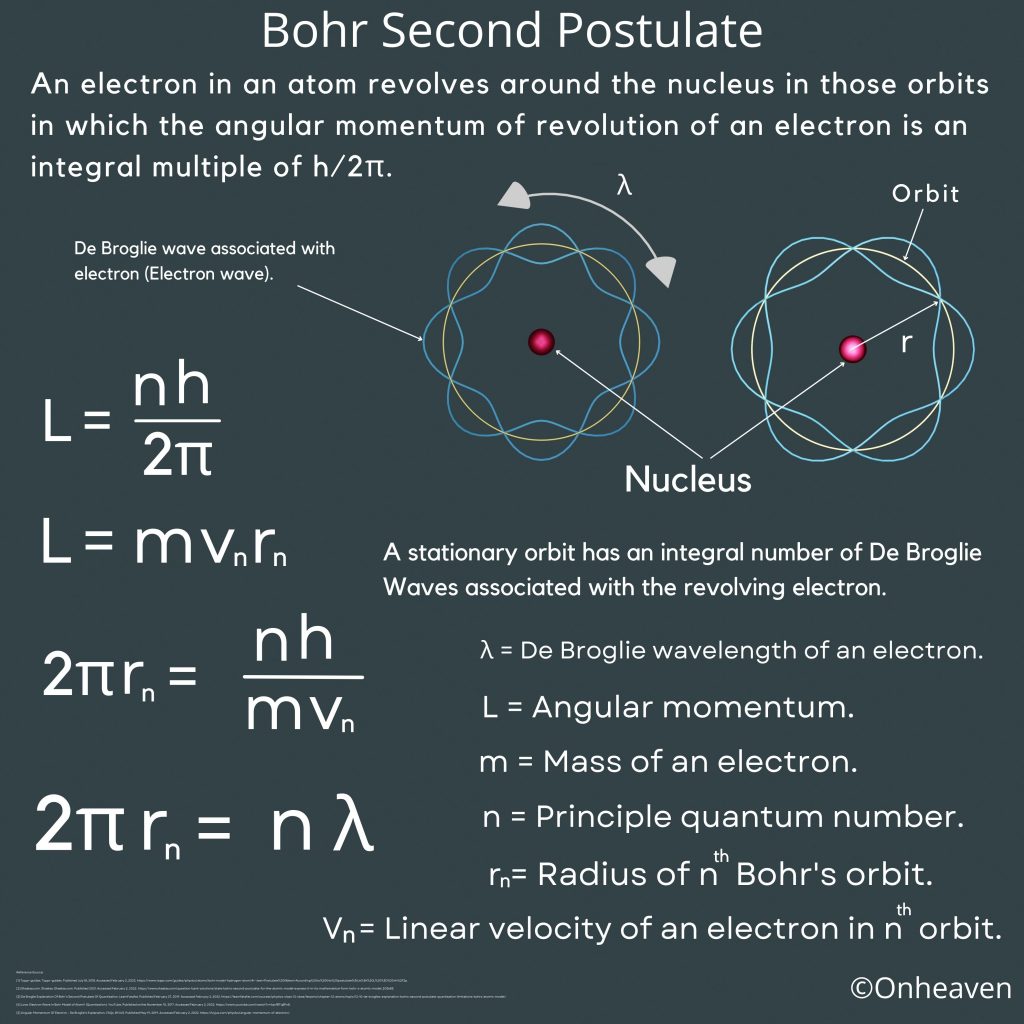
An electron in an atom revolves around the nucleus in those orbits in which the angular momentum of revolution of an electron is an integral multiple of h/2π.
A stationary orbit has an integral number of De Broglie Waves associated with the revolving electron.
Reference Source:
[1] Toppr-guides. Toppr-guides. Published July 18, 2018. Accessed February 2, 2022. https://www.toppr.com/guides/physics/atoms/bohr-model-hydrogen-atom/#:~:text=Postulate%20II&text=According%20to%20this%20postulate%3A,is%3A%20L%20%3D%20nh%2F2p.
[2] Shaalaa.com. Shaalaa. Shaalaa.com. Published 2021. Accessed February 2, 2022. https://www.shaalaa.com/question-bank-solutions/state-bohrs-second-postulate-for-the-atomic-model-express-it-in-its-mathematical-form-bohr-s-atomic-model_203683.
[3] De Broglie Explanation Of Bohr’s Second Postulate Of Quantisation. LearnFatafat. Published February 27, 2019. Accessed February 2, 2022. https://learnfatafat.com/courses/physics-class-12-cbse/lessons/chapter-12-atoms/topic/12-10-de-broglies-explanation-bohrs-second-postulate-quantisation-limitations-bohrs-atomic-model/.
[4] Love. Electron Wave in Bohr Model of Atom!! (Quantization). YouTube. Published online November 10, 2017. Accessed February 2, 2022. https://www.youtube.com/watch?v=4qxfBTqBFn8.
[5] Angular Momentum Of Electron – De Broglie’s Explanation, FAQs. BYJUS. Published May 19, 2019. Accessed February 2, 2022. https://byjus.com/physics/angular-momentum-of-electron/.