Avogadro’s law was hypothesized in the year 1811, by the Amedeo Avogadro.
Avagadro hypothesized that, at equal volume of different gasses at the same temperature and same pressure contains the equal number of molecules.
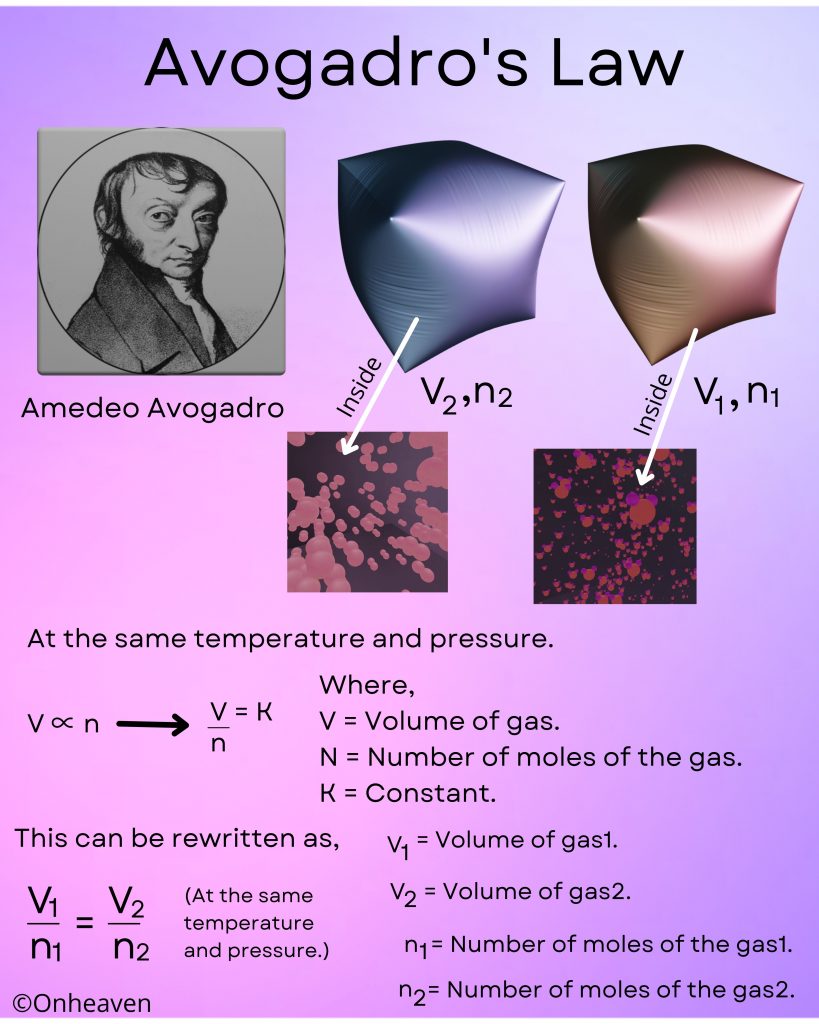
At constant temperature and constant pressure, volume and number of moles of gas are directly proportional to each other.
At the same temperature and pressure.
V∝n or V/n = K
Where,
V = Volume of gas.
N = number of moles of the gas.
K = Constant.
This can be rewritten as,
V1/n1 = V2/n2.
V1 = Volume of gas 1.
V2 = Volume of gas 2.
n1 = number of moles of gas 1.
n2 = number of moles of gas 2.
Or
V1/n1 = V2/n2.
V1 = Initial Volume of Gas.
n1 = Initial number of mole of Gas.
V2 = Final Volume of Gas.
n2 = Final number of mole of Gas.
Reference Source:
[1] Amedeo Avogadro. Science History Institute. Published June 2016. Accessed March 24, 2022. https://www.sciencehistory.org/historical-profile/amedeo-avogadro
[2] Amedeo Avogadro – Biography, Facts and Pictures. Famousscientists.org. Published 2014. Accessed March 24, 2022. https://www.famousscientists.org/amedeo-avogadro/
[3] Wikipedia Contributors. Amedeo Avogadro. Wikipedia. Published October 9, 2021. Accessed March 24, 2022. https://en.wikipedia.org/wiki/Amedeo_Avogadro
[4] Wikipedia Contributors. Avogadro’s law. Wikipedia. Published July 12, 2021. Accessed March 24, 2022. https://en.wikipedia.org/wiki/Avogadro%27s_law
[5] Amedeo Avogadro | Biography, Law, Discoveries, & Facts | Britannica. In: Encyclopædia Britannica. ; 2022. Accessed March 24, 2022. https://www.britannica.com/biography/Amedeo-Avogadro
[6] Biography of Amedeo Avogadro, Influential Italian Scientist. ThoughtCo. Published 2019. Accessed March 24, 2022. https://www.thoughtco.com/amedeo-avogadro-biography-606872