Like all elementary particles, electrons exhibit properties of both particles and waves: they can collide with other particles and can be diffracted like light.
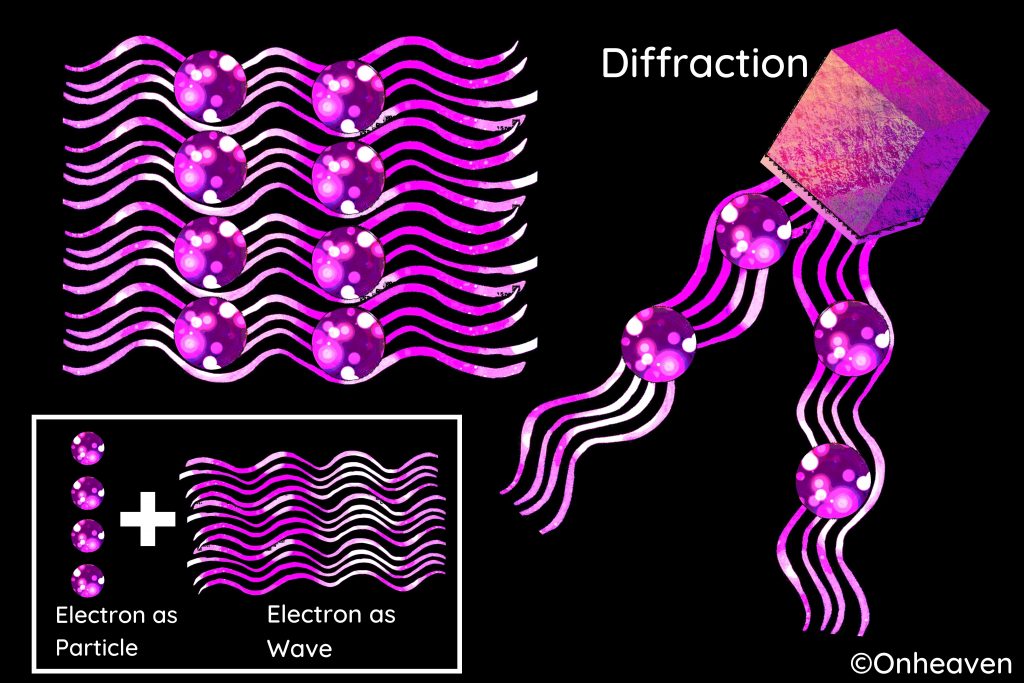
😊Electrons are involved in numerous applications such as electronics, welding, cathode ray tubes, electron microscopes, radiation therapy, lasers, gaseous ionization detectors and particle accelerators.
😊 The electron is a subatomic particle, symbol e− or β−, whose electric charge is negative one elementary charge.
😊Electrons belong to the first generation of the lepton particle family, and are generally thought to be elementary particles because they own no familiar components or substructure.

😊The antiparticle of the electron is called the positron it is identical to the electron except it carries electrical and other charges of the opposite sign.
😊The Coulomb force interaction between the positive protons in reach atomic nuclei and the negative electrons without, allows the composition of the two familiar as atoms.
😊Laboratory instruments are capable of trapping individual electrons as well as electron plasma by the use of electromagnetic fields.
😊Electrons can also participate in nuclear reactions, such as nucleosynthesis in stars, where they are familiar as beta particles.
😊When an electron collides with a positron, both particles can be annihilated, producing gamma ray photons.
😊Interactions involving electrons with other subatomic particles are of interest in fields such as chemistry and nuclear physics.
😊Electrons play an essential role in numerous physical phenomena, such as electricity, magnetism, chemistry and thermal conductivity, and they also participate in gravitational, electromagnetic and weak interactions.

😊Quantum mechanical properties of the electron consist of an intrinsic angular momentum of a half-integer value, expressed in units of the reduced Planck constant, ħ.
😊Ionization or differences in the proportions of negative electrons versus positive nuclei changes the binding energy of an atomic system.
😊In 1838, British natural philosopher Richard Laming first hypothesized the idea of an indivisible quantity of electric charge to explain the chemical properties of atoms.
😊Electromagnetic fields produced from other sources will affect the motion of an electron according to the Lorentz force law.
😊The exchange or sharing of the electrons between two or additional atoms is the main cause of chemical bonding.

😊Electrons can be along with through beta decay of radioactive isotopes and in high-energy collisions, in support of instance when cosmic rays enter the atmosphere.
😊Electrons radiate or absorb energy in the form of photons when they are accelerated.
😊Being fermions, no two electrons can occupy the same quantum state, in accordance with the Pauli exclusion principle.
😊The electron has a mass is 9.109×10−31 kilograms which is approximately 1/1836 of the proton.

Source:
[1] Wikipedia Contributors. “Electron.” Wikipedia, Wikimedia Foundation, 6 Nov. 2020, en.wikipedia.org/wiki/Electron. Accessed 7 Nov. 2020.
[2] pixy.org. “Electron Lightning Drawing Image.” Pixy.org, 12 May 2020, pixy.org/5944917/. Accessed 7 Nov. 2020.
“In 2018 Adrian Ferent discovered mass–energy equivalence for Dark Matter E = md × vp2, added it to E = mc2 and Ferent’s mass–energy equivalence equation: E= mc2 + md × vp2”
Adrian Ferent
“In electron and positron collision Gamma rays are created because electrons and positrons are made of Gamma rays”
Adrian Ferent
“Because in Ferent Quantum Gravity the electrons are made of Gamma rays and Dark Matter, is the first proofs for the mass–energy equivalence equation: E = mc2 “
Adrian Ferent
Comments are closed.