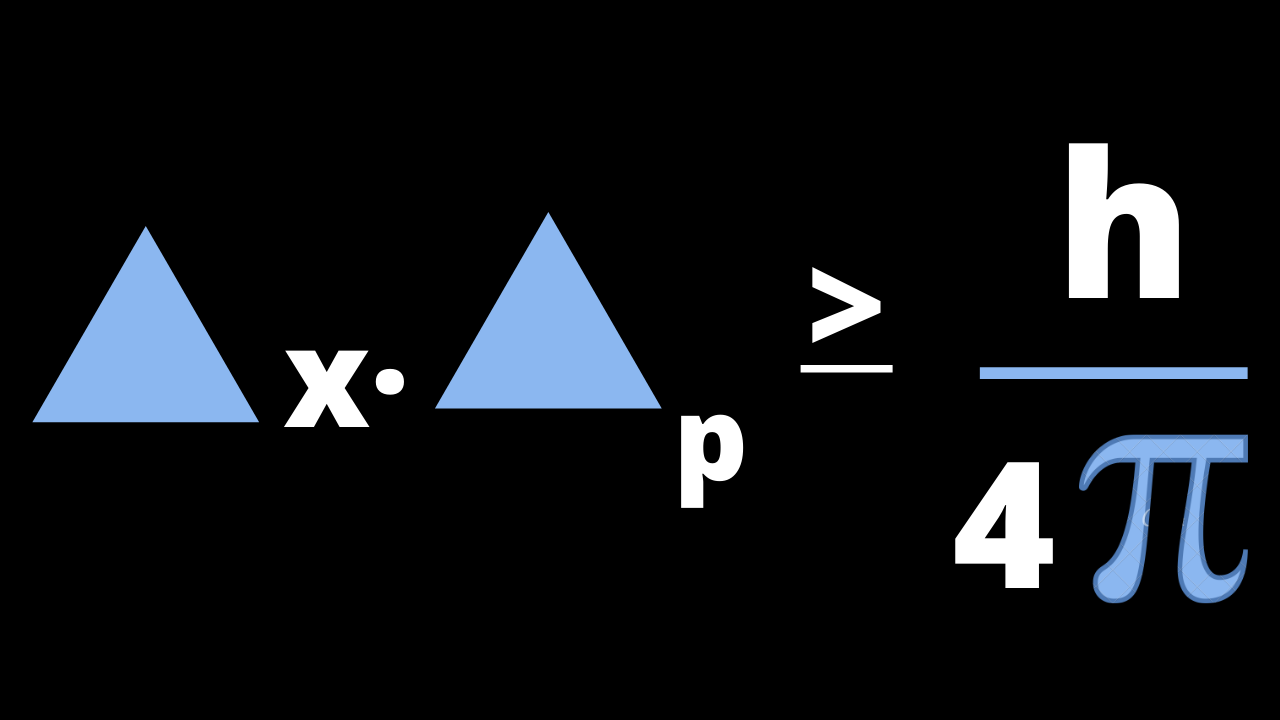Key Idea: We can’t measure the exact position and momentum at the same time of any particle.
Representation of the formula of the Heisenberg’s uncertainty principle of the particle:
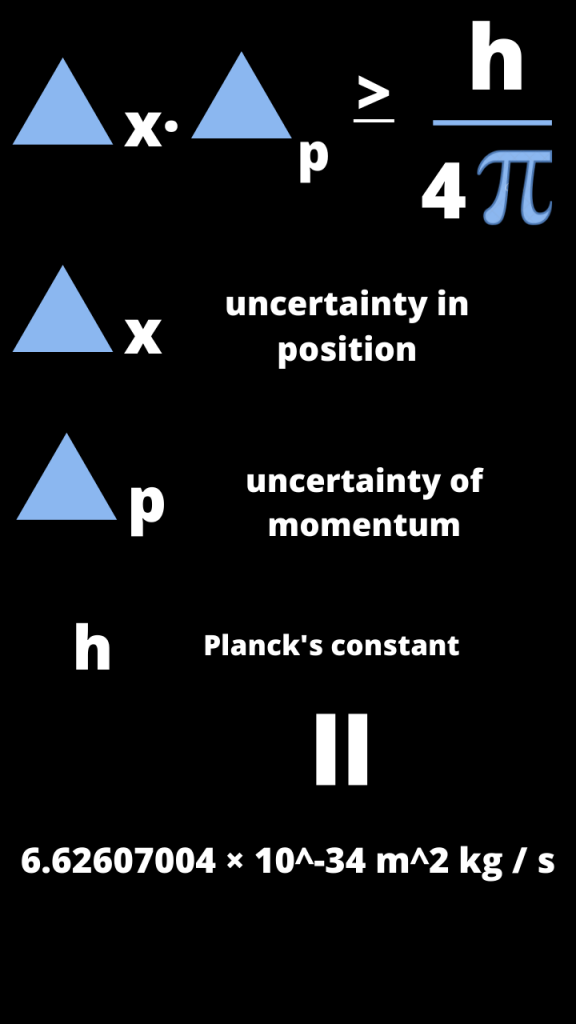
According to this formula the position and momentum of any particle is inversely correlated to each other.
As we became more and more certain in position, then at same time we became more and more uncertain about momentum of particle and vice-versa.
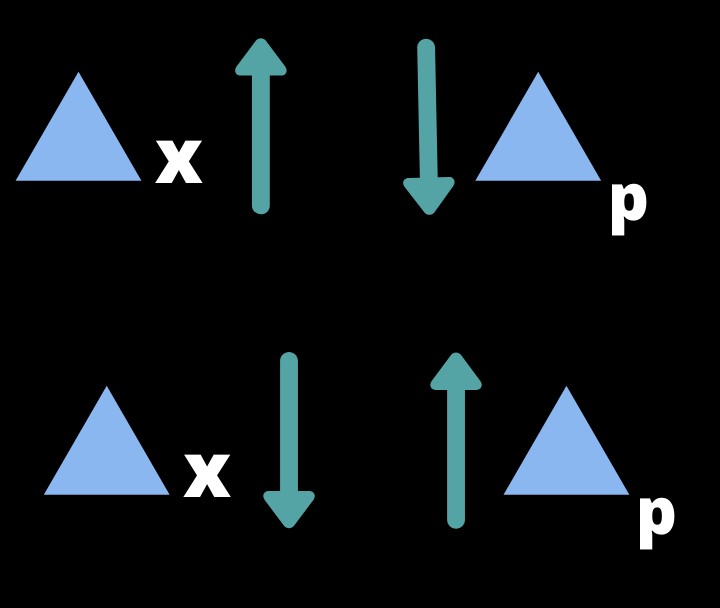

Here is the practical experiment video of this principle:
Source:
Wikipedia Contributors. “Uncertainty Principle.” Wikipedia, Wikimedia Foundation, 31 Oct. 2020, en.wikipedia.org/wiki/Uncertainty_principle. Accessed 13 Nov. 2020.