
Photon energy is the energy carried by a single photon.
The amount of energy is directly proportional to the photon’s electromagnetic frequency and thus, equivalently, is inversely proportional to the wavelength.
Photon energy can be expressed using any unit of energy.
The higher the photon’s frequency, the higher its energy.
Equivalently, the longer the photon’s wavelength, the lower its energy.
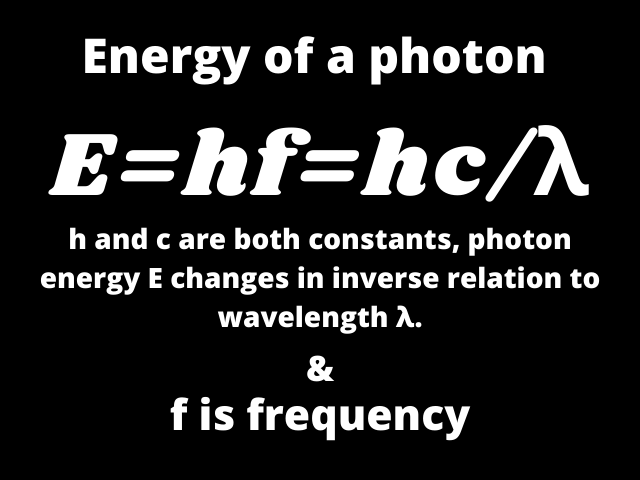

As h and c are both constants, photon energy E changes in inverse relation to wavelength λ.
Substituting h with its value in J⋅s and f with its value in hertz gives the photon energy in joules.
Therefore, the photon energy at 1 Hz frequency is 6.62606957 × 10−34 joules or 4.135667516 × 10−15 eV.
Source:
[1] Wikipedia Contributors. “Photon Energy.” Wikipedia, Wikimedia Foundation, 30 Oct. 2020, en.wikipedia.org/wiki/Photon_energy. Accessed 12 Nov. 2020.
[2] “Photon Energies and the Electromagnetic Spectrum | Physics.” Lumenlearning.com, 2020, courses.lumenlearning.com/physics/chapter/29-3-photon-energies-and-the-electromagnetic-spectrum/. Accessed 12 Nov. 2020.
[3] “Physics 342: Useful Constants.” Rutgers.Edu, 2011, http://www.physics.rutgers.edu/~abrooks/342/constants.htmlAccessed 12 Nov. 2020.
[4] geralt. “Physics Quantum Particles – Free Image on Pixabay.” Pixabay.com, 10 Feb. 2020, pixabay.com/illustrations/physics-quantum-physics-particles-4835310/. Accessed 12 Nov. 2020.